Model-based development of synthetic yeast communities
The conversion of lignocellulosic materials to commodity chemicals requires the efficient fermentation of sugar monomers to products. Co-culturing different species of yeast has been shown to be an effective strategy for fermentation of sugar mixtures. For example, co-culturing a respiratory deficient mutant of the ethanologic yeast Sacchromyces cerevisiae with the xylose-fermenting yeast Scheffersomyces stipitis has been shown to produce larger amounts of ethanol from glucose and xylose mixtures than either yeast in monoculture. However, the processes commonly used in the pretreatment and hydrolysis of sugar polymers produce dehydration products such as furfural and 5-hydroxymethyl furfural (HMF) that have a deleterious effect on fermentative microorganisms. Many microbes have the capacity to reduce these furan compounds to less toxic alcohols. For example, HMF is reduced by S. cerevisiae at approximately a quarter of the rate that furfural is reduced. Although S. stipitis can detoxify HMF at a faster rate than S. cerevisiae, furan aldehydes have a more deleterious effect on S. stipitis growth and ethanol production.
We have combined genome-scale models of S. cerevisiae and S. stipitis metabolism with batch monoculture and co-culture experiments to develop improved understanding of furaldehyde detoxification by these yeast species. Uptake kinetics and stoichiometric equations for the intracellular reduction reactions associated with each inhibitor were added to genome-scale metabolic reconstructions of the two yeasts. Further modification of the S. cerevisiae metabolic network was necessary to satisfactorily predict the amount of acetate synthesized during HMF reduction. Inhibitory terms that captured the adverse effects of the furan aldehydes and their corresponding alcohols on cell growth were added to attain qualitative agreement with batch experiments. When the two yeasts were co-cultured in the presence of the furan aldehydes, inoculums that reduced the synthesis of highly toxic acetate produced by S. cerevisiae yielded the highest ethanol productivities. Our model can be used to generate optimal cellular engineering and fermentation strategies for the simultaneous detoxification and fermentation of lignocellulosic hydrolysates by S. cerevisiae and/or S. stipitis.
Funding:UMass Institute for Cellular Engineering, ReCommunity Recycling
Student: Timothy Hanly (5th year Ph.D. student)
Recent Publications:
- Kambam, P. K. R. and M. A. Henson, "Engineering Bacterial Processes for Cellulosic Ethanol Production," Biofuels, 1, 729-744 (2010).
- Hanly, T. J. and M. A. Henson, "Dynamic Flux Balance Modeling of Microbial Co-Cultures for Efficient Batch Fermentation of Glucose and Xylose Mixtures," Biotechnology and Bioengineering, 108, 376-385 (2010). [PDF]
- Hanly, T. J., M. Urello and M. A. Henson, “Dynamic Flux Balance Modeling of S. cerevisiae and E. coli Co-cultures for Efficient Consumption of Glucose/Xylose Mixtures,” Applied Microbiology and Biotechnology, 93, 2529-2541 (2012). [PDF]
- Mahadevan, R. and M. A. Henson, “Genome-based Modeling and Design of Metabolic Interactions in Microbial Communities,” Computational and Structural Biotechnology Journal, 3: e201210008. dx.doi.org/10.5936/csbj.201210008 (2012).[PDF]
- Hanly, T. J. and M. A. Henson, “Dynamic Metabolic Modeling of a Microaerobic Yeast Co-culture: Predicting and Optimizing Ethanol Production from Glucose/Xylose Mixtures,” Biotechnology for Biofuels, 6, 44, doi:10.1186/1754-6834-6-44 (2013). [PDF]
- 6. Hanly, T. J. and M. A. Henson, “Dynamic Metabolic Modeling of a Microaerobic Yeast Co-culture: Predicting and Optimizing Ethanol Production from Glucose/Xylose Mixtures,” submitted for publication.

|
|
|
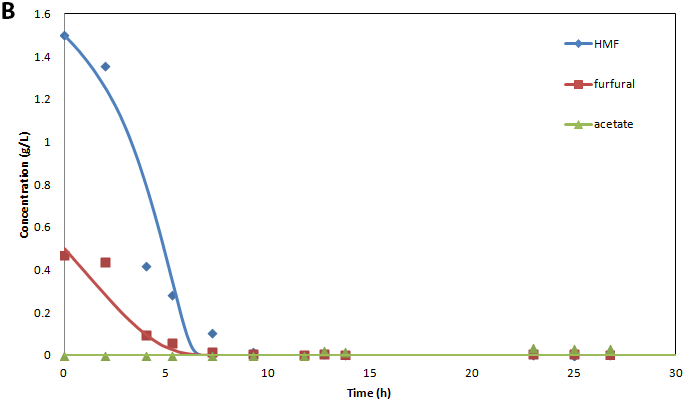
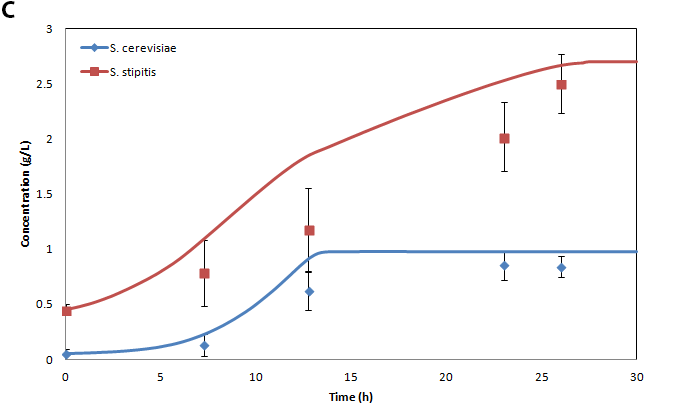
A) Metabolite and biomass concentrations, B) inhibitor concentrations, and C) individual cell counts for a co-culture initiated with an inoculum of 10% S. cerevisiae 311 and 90% S. stipitis consuming 14.5 g/L glucose and 8 g/L xylose in the presence of 0.9 g/L furfural 1.85 g/L HMF. Points represent experimental measurements and lines represent model predictions.