Downstream Pharmaceutical Manufacturing
Downstream processing of Active Pharmaceutical Ingredients (APIs) typically involves several batch processing steps such as extraction, crystallization, filtration and drying. Removal of the solid API from liquid solvents via filtration and drying operations often represents a bottleneck in the manufacturing process. For example, drying is typically the rate limiting step in the overall manufacturing cycle and is by far the most energy intensive unit operation. Both filtration and drying can adversely impact API properties if operating conditions are not carefully selected and controlled. In vacuum drying, improper combinations of temperature and pressure can lead to API degradation that affects drug efficacy. Filtration and drying traditionally have been performed in different pieces of equipment. To reduce processing times and minimize material transfer losses, the two processing steps can be combined into a single unit operation termed as an agitated filter dryer. While more potentially more effective and efficient, these units offer their own challenges including the possibility of API attrition and agglomeration due to mixing shear forces.
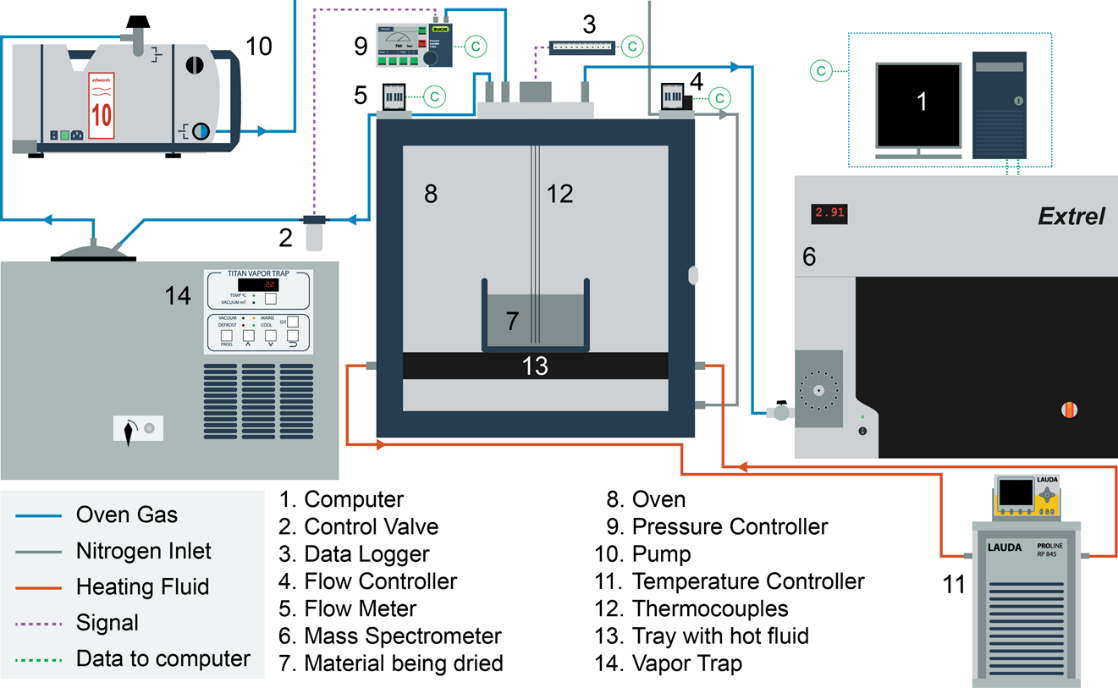
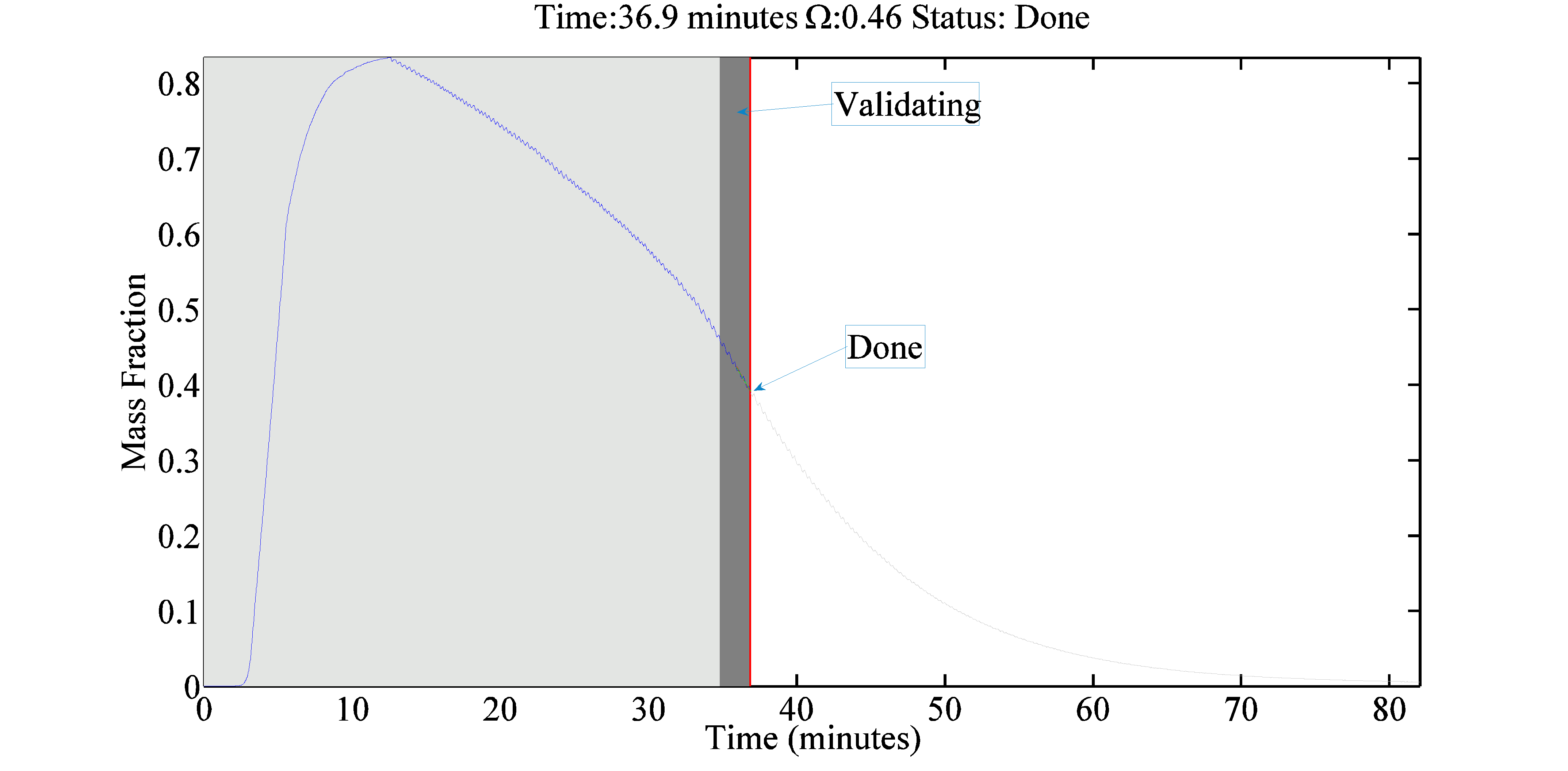
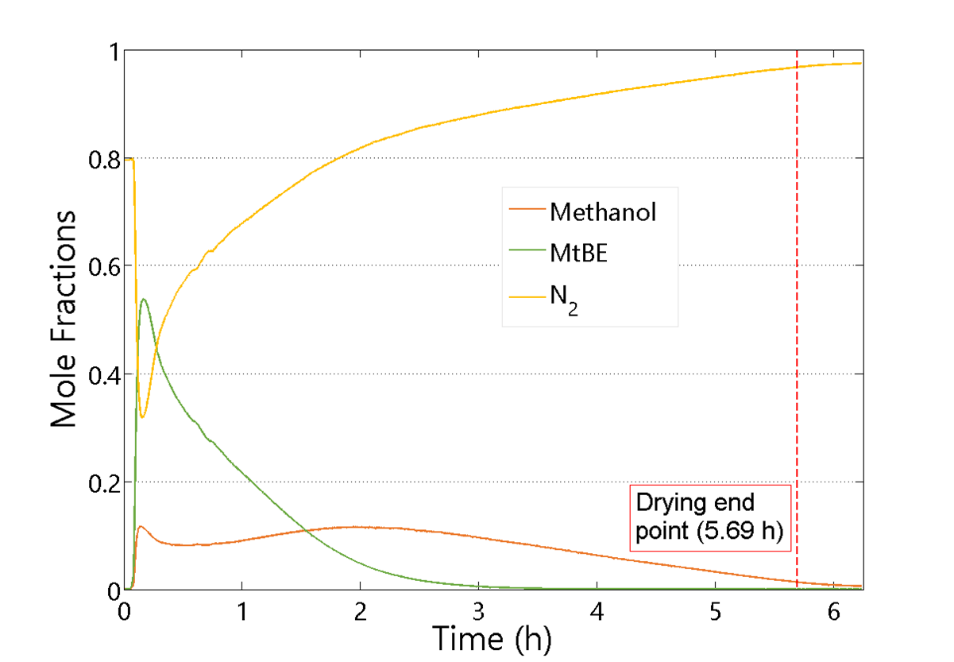
We are developing dynamic process models for API filtration and drying operations. Our initial work has focused on vacuum drying, including the formulation of combined heat-mass transport models and the development of a novel method for determining the drying end point based on on-line mass spectroscopy. The end detection algorithm was successfully tested on laboratory-scale and manufacturing-scale vacuum dryers by comparing model predictions to loss on drying measurements. Our current work is focused on incorporating the API particle size distribution, agitation rate and cake compaction effects into filtration and drying process models. Dynamic simulations are performed to provide insights into key design parameters and to enable process improvement and optimization.
Project funding is generously provided by:
Current projects include:
- Development of agitated filter dryer models that predict API particle attrition and agglomeration as well as drying performance under different drying and mixing conditions.
Collaborator: Kostas Saranteas, Sunovion Pharmaceuticals
- Development of pressure filtration models that predict the effects of the API particle size distribution and cake compaction on filtration performance for different suspensions and applied pressures.
Collaborator: Kostas Saranteas, Sunovion Pharmaceuticals
Selected publications:
- Dodda, A. G., K. Saranteas and M. A. Henson, “Using On-Line Mass Spectrometry to Predict the End Point during Drying of Pharmaceutical Products,” Organic Process Research and Development, 19, 122-131, doi: 10.1021/op400272t (2015). [Link]
- Dodda, A. G., K. Saranteas and M. A. Henson, “Multiphase Transport Modeling for Vacuum Drying of Pharmaceutical Products,” AIChE Journal, 61, 3639-3655, doi: 10.1002/aic.14879 (2015). [PDF]
- Dodda, A. G., K. Saranteas and M. A. Henson, “The Effect of Continuous Agitation and Solvent Concentration on API Attrition During Vacuum Agitated Filter Drying.” [Submitted]