|
CEE 680 |
|
18 December 2007 |
FINAL EXAM
Closed book, three pages of notes allowed.
Answer Question A and either B or C. Please state any additional assumptions you made, and show all work. If you don’t have time to complete a section, please describe how you would solve the problem (without using a computer program such as MINEQL).
Part I: Answer Question A
A. Solubility & Predominance. (75%)
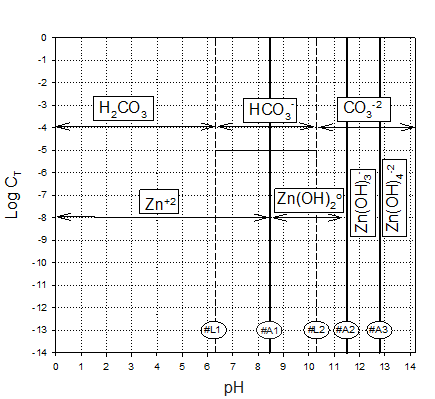
Zinc carbonate (ZnCO3(s)) and Zinc Hydroxide (Zn(OH)2 (s)) are two important solid phases that may control zinc solubility in water[1]. In the attached pages is a detailed solution leading to a zinc hydroxide solubility diagram[2]. Please use this to help solve the following problems.
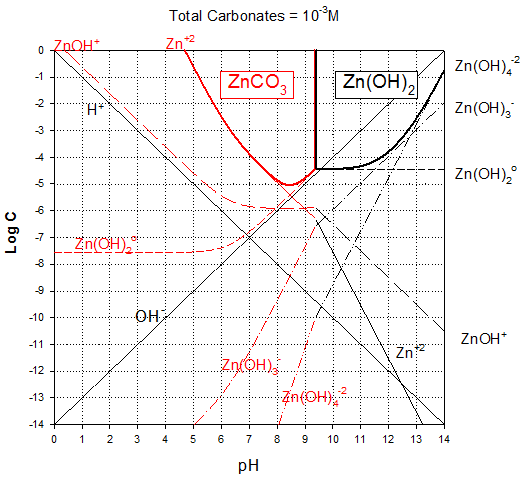
1. Prepare a solubility diagram (log C vs pH) for a water that is potentially in equilibrium with zinc hydroxide and zinc carbonate. Assume the water has 10-3 M total carbonates. Show all soluble species along with the ZnT line and indicate where precipitation will occur and the type of precipitate. Please feel free to use any of the hydroxide calculations in developing the answer to part 1 or part 2. It could save you some time.
2. Prepare a predominance diagram, showing the precipitates and major soluble species (in areas where there are no precipitates). As would be typical for a problem of this type, make pH the x-axis, and log total carbonate (CO3T), the y-axis. Assume a total soluble zinc concentration of 10-3 M (1 mM). Again you may find that using the solubility diagram provided or the one you did for part 1 can help in directing your work for part 2.
|
Equilibrium[3] |
Log K |
|
Zn |
-5.04 |
|
Zn(OH)2o = ZnOH+ + |
-6.06 |
|
Zn(OH)3-1 = Zn(OH)2o
+ |
-2.50 |
|
Zn(OH)4-2 = Zn(OH)3-1
+ |
-1.20 |
|
Zn(OH)2 (s) = Zn+2 + 2OH- |
-15.55 |
|
ZnCO3 (s) = Zn+2 + CO3-2 |
-10.26 |
Development of hydroxide solubility diagram.
a. Using the
equilibria for zinc hydroxide from above:
![]()
we get the following for the free aquo ion:
![]()
![]()
![]()
From this we use the hydroxide equilibria to get hydroxide species concentrations:
and for the monohydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
now for the dihydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
now for the trihydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
now for the tetrahydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
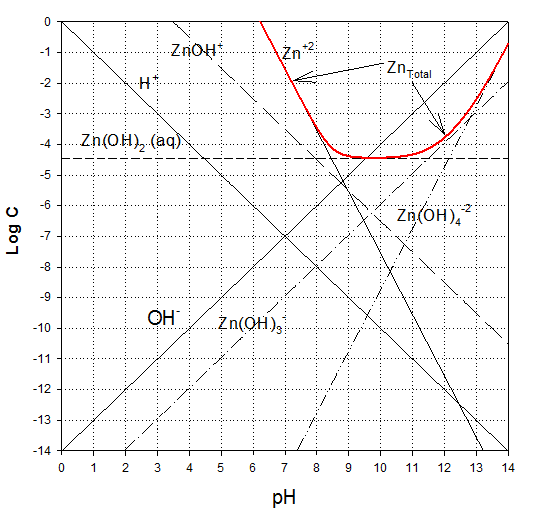
Answer to A.1.
And for the carbonate, we must consider:
|
ZnCO30 = Zn+2 + CO3-2 |
-5.30 |
|
Zn(OH)2 (s)
= Zn+2 + 2 |
-15.55 |
|
ZnCO3 (s) = Zn+2 + CO3-2 |
-10.26 |
b. Using the equilibria for zinc carbonate from
above:
![]()
b1. we get the
following for the free aquo ion:
![]()
![]()
And for 10 mM total carbonates:
![]()
![]()
For Carbonate Calculations:
For pH<6.3, ![]() , or
, or ![]()
For pH=6.3-10.3, ![]() , or
, or ![]()
For pH>10.3, ![]() , or
, or ![]()
So for pH<6.3, we have:
![]()
![]()
![]() , @pH<6.3
, @pH<6.3
And for pH=6.3-10.3, we have:
![]()
![]()
![]() , @pH=6.3-10.3
, @pH=6.3-10.3
And for pH>10.3, we have:
![]()
![]()
![]() , @pH>10.3
, @pH>10.3
b2. and for the monohydroxide:
from the Zn(OH)2(s) based calculations, we know that:
![]()
And now substituting in the ZnCO3(s)-based free zinc equation for pH<6.3, we have:
![]()
![]() , @pH<6.3
, @pH<6.3
And for pH=6.3-10.3, we have:
![]()
![]() , @pH=6.3-10.3
, @pH=6.3-10.3
And for pH>10.3, we have:
![]()
![]() , @pH>10.3
, @pH>10.3
b3. now for the dihydroxide:
from the Zn(OH)2(s)-based calculations, we know that:
![]()
And now substituting in the ZnCO3(s)-based ZnOH equation for pH<6.3, we have:
![]()
![]() , @pH<6.3
, @pH<6.3
And for pH=6.3-10.3, we have:
![]()
![]() , @pH=6.3-10.3
, @pH=6.3-10.3
And for pH>10.3, we have:
![]()
![]() , @pH>10.3
, @pH>10.3
B4. now for the trihydroxide:
from the Zn(OH)2(s)-based calculations, we know that:
![]()
And now substituting in the ZnCO3(s) based Zn(OH)2 equation for pH<6.3, we have:
![]()
![]() , @pH<6.3
, @pH<6.3
And for pH=6.3-10.3, we have:
![]()
![]() , @pH=6.3-10.3
, @pH=6.3-10.3
And for pH>10.3, we have:
![]()
![]() , @pH>10.3
, @pH>10.3
b5. now for the tetrahydroxide:
from the Zn(OH)2(s)-based calculations, we know that:
![]()
And now substituting in the ZnCO3(s) based Zn(OH)3 equation for pH<6.3, we have:
![]()
![]() , @pH<6.3
, @pH<6.3
And for pH=6.3-10.3, we have:
![]()
![]() , @pH=6.3-10.3
, @pH=6.3-10.3
And for pH>10.3, we have:
![]()
![]() , @pH>10.3
, @pH>10.3
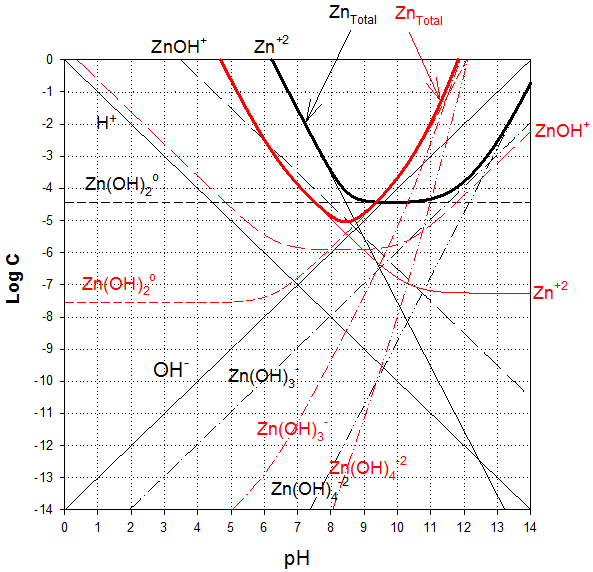
Now combining, we find that the hydroxide controls at pHs of 12.7 and above. Below this level, the gypsum is less soluble.
Answer to A.2.
Type A lines
First, lets consider the boundary between the aquo ion and the monohydroxide
![]()
When they’re present at equal concentrations:
![]()
or:
![]()
And this gives us A1:
pH = 8.96
following the same approach, we get the remaining A lines
![]()
Gives us A2:
pH =7.94
but since this is less than A1, it tells us that the monohydroxide never predominates, so we need to look at the boundary between the free zinc and the dihydroxide
![]()
![]()
At the boundary, ![]()
![]()
Gives us the revised A1:
pH =8.45
![]()
Gives us the revised A2:
pH =11.50
![]()
Gives us A3:
pH =12.80
Type B line
First let’s examine the hydroxide equilibria. By looking at the Zn(OH)2 solubility graph, its appears that only free zinc (Zn+2) and the tetrahydroxide (Zn(OH)4-2) are the dominant soluble species in equilibrium with the hydroxide precipitate at a total soluble zinc concentration of 10-3 M.
So, first let’s look at the Zn+2/Zn(OH)2 precipitate boundary. From the solubility product equation:
![]()
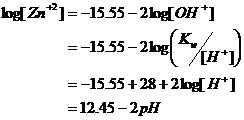
Of course, this is just a re-derivation of the equation that was already provided as part of the hydroxide solubility calculations:
![]()
now adopting a total Zn of 1 mM, we get:
![]()
or:
pH =7.725
this line is valid as the A lines clearly show Zn+2 to be dominant at this pH
Now let’s look at the Zn(OH)4-2/Zn(OH)2 precipitate boundary. From the given solubility calculations, we have:
![]()
Once again, adopting a total Zn of 1 mM, we get:
![]()
or:
pH =12.875
This falls just above the A3 line, so its also valid
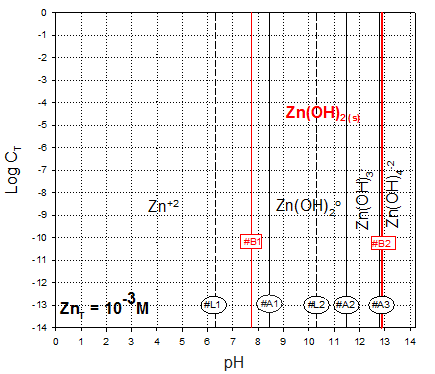
Inspection of the carbonate/hydroxide solubility diagram leads one to conclude that free zinc is the only soluble species that needs to be considered for the B lines defining carbonate solubility.
So, lets look at the Zn+2/ZnCO3 boundary: From the solubility calculations we had developed three line segments. We can conveniently take the equation for each, retaining the total carbonate term in its general form:
B3a:
For pH<6.3, we had:
![]()
And setting the free zinc equation to 1 mM, we get
![]()
![]() , @pH<6.3
, @pH<6.3
B3b:
And for pH=6.3-10.3, we have:
![]()
![]()
![]() , @pH=6.3-8.45
, @pH=6.3-8.45
However, this is only valid up to pH 8.45, the limit of free zinc’s predominance (A1 line)
We could go on and graph the lines defining the border between the various zinc hydroxides, but its clear that they won’t be necessary. This was determined from inspection of the carbonate/hydroxide solubility graph. Its also clear from the emerging predominance diagram (above). The zinc carbonate lines already extend into the zinc hydroxide precipitate zone. This border is defined by type C lines.
Type C line
Finally, lets consider the boundary between the two solid phases. This line will start on the low pH side, were the B lines intersect (pH=7.725). This is well within the zone where bicarbonate is the dominant carbonate species.
First, from the hydroxide solid solubility as given, we have:
![]()
And from the carbonate solubility we had calculated:
C1b:
For the bicarbonate zone; pH=6.3-10.3:
![]()
![]()
Substituting in the hydroxide-based equation, we get:
![]()
![]() , @pH=6.3-10.3
, @pH=6.3-10.3
C1c:
And for the carbonate zone; pH>10.3, we determined:
![]()
Substituting in the hydroxide-based equation, we get:
![]()
![]()
![]() , @pH>10.3
, @pH>10.3
The complete lines are as follows:
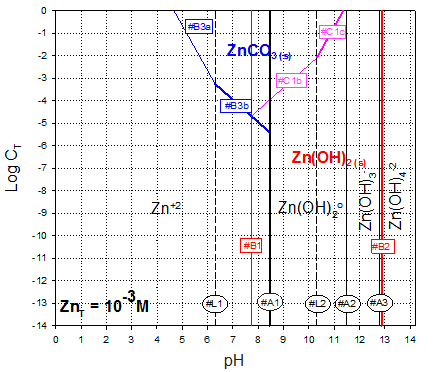
And removing extra line segments and zinc species that do not exist at 1 mM concentration (e.g., those “under” the precipitates, we get the final diagram:
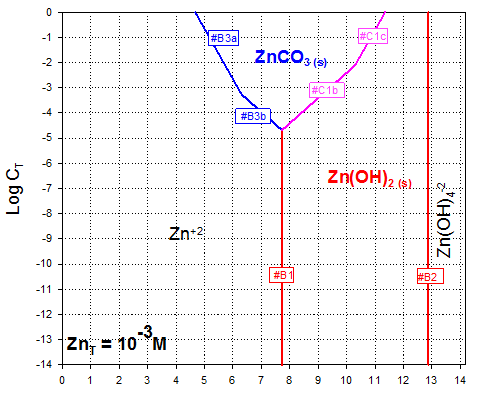
Answer to A.3.
If enough carbonate was present, the calcium solubility would be depressed, especially at high pHs. There would also be a calcium carbonate phase zone in predominance diagrams.
Part II:. Answer either B or C
B. Redox (25%)
Galvanized pipe is still quite common in home plumbing in the US and Europe. The zinc coating on these pipe materials can corrode and undergo oxidation from the zero-valent metal to the soluble divalent aqueous zinc.
Residual active chlorine (hypochlorous acid) is a powerful oxidant. It can readily cause the oxidation of zinc metal to the divalent cation. However, in many drinking water systems, chlorine is not used as a disinfectant (e.g., groundwater systems) or it becomes substantially dissipated. The major residual oxidant is dissolved oxygen. After reaction, xxygen’s final reduced form is usually hydroxide and water. You have been asked to comment on the possibility that zinc can be oxidized by oxygen, and whether oxidized zinc can affect other metals. Specifically, your tasks are:
1. Write a balanced equation for the oxidation of metallic zinc to Zn+2 by dissolved oxygen
2. Determine the stoichiometry of this reaction (e.g., mg-O2/mg-Zn).
3. Determine the equilibrium constant (K) for this reaction and comment on what thermodynamics tells you about whether this is a favorable reaction.
4. Make a similar determination about the abililty of oxidized zinc (Zn+2 ) to oxidize metallic iron to Fe+2.
Answer to B.1.
Use the following redox constants:
|
Equ# |
Half Cell Reaction |
DEo (Volts) |
|
12 |
Zn+2 +
2e- = Zn(s) |
-0.76 |
|
1 |
O2(g) + 4H+ + 4e- = 2H2O |
+1.23 |
|
17 |
Fe+2 + 2e- = Fe(s) |
-0.44 |
|
Factor |
Equ# |
Half
Cell Reaction |
DEo (Volts) |
|
¼ |
1 |
¼O2(g)
+ H+ + e- = ½H2O |
+1.23 |
|
-½ |
12 |
½Zn(s) = ½Zn+2 +
e- |
+0.76 |
|
|
|
¼O2(g)
+ H+ + ½Zn(s) = ½Zn+2 + ½H2O |
+1.99 |
Or simplifying by making it per zinc atom:
½O2(g) + 2H+ + Zn(s)
= Zn+2 + H2O
Answer to B.2.
Stoichiometry:
![]()
![]()
![]()
Answer to B.3.
![]()
If we define the equation as:
½O2(g) +
2H+ + Zn(s) = Zn+2 + H2O
This si a 2-electron transfer, so:
![]()
![]()
This means that following is true at equilibrium:
![]()
Since the partial pressure of oxygen is about 0.2, and the typical hydrogen ion concentration is 10-7, the equilibrium divalent zinc concentration would be greater than 10+50 M. In other words, this is a reaction that essentially goes to completion, from the standpoint of thermodynamics.
Answer to B.4.
For the reaction of zinc and iron:
|
Factor |
Equ# |
Half
Cell Reaction |
DEo (Volts) |
|
1 |
12 |
Zn+2 +
2e- = Zn(s) |
-0.76 |
|
-1 |
17 |
Fe(s)
= Fe+2 + 2e- |
+0.44 |
|
|
|
Zn+2 +
Fe(s) = Zn(s)
+ Fe+2 |
-0.32 |
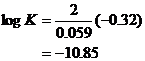
![]()
The time the amout of products is extremely small compared to the amount of reactants, so the reaction must be considered to be highly unfavorable. Hydrolysis of the metals will not have a very large effect either.
C. Redox True/False (25%) Mark each one of
the following statements with either a "T" or an "F"
a. ___ F_ Lead is toxic partly because it has the same redox potential as calcium .
b. ___ T_ As metals become more oxidized, they tend to be more prone to hydrolysis
c. ___ T_ Most Redox reactions essentially result in complete conversion to products or no conversion at all when at equilibrium
d. ___ F_ Phosphate helps control corrosion by chemically reducing certain oxidized metal species
e. ___ F_ Environmental systems are far more likely to be at redox (e.g., electron) equilibrium than at acid/base (e.g., proton) equilibrium.
f. ___ F_ Oxidation state is almost always changes with ligand number.
g. ___ T_ The oxidation state of phosphorus in the environment is almost always +V.
h. ___ F_ As pe increases, so does the electron activity
i. ___ F_ The easiest way to measure half cell potentials is with a standard hydrogen electrode.
j. ___ T_ For the ferrous/ferric redox couple, the value of ao plus a1 must always equal one.
Some important equilibrium constants:
|
Equilibria |
Log K |
α0 at pH 9 |
|
Mg(OH)2 (s) = Mg+2 + 2OH- |
-11.6 |
|
|
Fe+3 + H2O = FeOH+2 + H+ |
-2.19 |
|
|
Mg+2 + H2O = MgOH+ + H+ |
-11.44 |
0.9964 |
|
MgCO3 (s) = Mg+2 + CO3-2 |
-7.5 |
|
|
CaCO3(s) = Ca+2 + CO3-2 |
-8.34 |
|
|
Ca(OH2)(s) = Ca+2 + 2OH- |
-5.19 |
|
|
CaSO4.2H2O(s)
= Ca+2 + SO4-2 + 2H2O |
-4.62 |
|
|
CaOH+ = Ca+2 + |
-1.15 |
0.9999 |
|
AlOH+2 = Al+3 + |
-9.01 |
0.0001 |
|
CdOH+ = Cd+2 + |
-3.92 |
0.9232 |
|
Co |
-4.80 |
0.6131 |
|
Cu |
-6.00 |
0.0909 |
|
FeOH+ = Fe+2 + |
-4.50 |
0.7597 |
|
Hg |
-10.60 |
0.0000 |
|
NiOH+ = Ni+2 + |
-4.14 |
0.8787 |
|
Pb |
-6.29 |
0.0488 |
|
Zn |
-5.04 |
0.4770 |
|
Zn(OH)2o = ZnOH+ + |
-6.06 |
|
|
Zn(OH)3-1 = Zn(OH)2o
+ |
-2.50 |
|
|
Zn(OH)4-2 = Zn(OH)3-1
+ |
-1.20 |
|
|
ZnCO30 = Zn+2 + CO3-2 |
-5.30 |
|
|
Zn(OH)2 (s)
= Zn+2 + |
-15.55 |
|
|
ZnCO3 (s) = Zn+2 + CO3-2 |
-10.26 |
|
Some
important half-cell reactions
|
Equ# |
Half
Cell Reaction |
DEo (Volts) |
Log K |
pe |
|
1 |
O2(g) + 4H+ + 4e- = 2H2O |
+1.23 |
|
|
|
2 |
Mn+3 +
e- = Mn+2 |
+1.51 |
|
|
|
3 |
Mn+4 +
e- = Mn+3 |
+1.65 |
|
|
|
4 |
MnO4- + 8H+ + 5e- = Mn+2 + 4H2O |
+1.49 |
|
|
|
5 |
Fe+3 +
e- = Fe+2 |
+0.77 |
|
|
|
6 |
Cu+2 + e- = Cu+ |
+0.16 |
|
|
|
7 |
½HOBr + ½H+ + e- =
½Br- + ½H2O |
+1.33 |
|
|
|
8 |
O3 (g) + 2H+ + 2 e- = O2 (g) + H2O |
+2.07 |
|
|
|
9 |
Al+3 + 3e- = Al(s) |
-1.68 |
|
|
|
10 |
S(s) + 2H+ + 2e- = H2S (g) |
+0.17 |
|
|
|
11 |
½NH2Cl + H+ +e-
= ½Cl- + ½NH4+ |
+1.40 |
|
|
|
12 |
Zn+2 +
2e- = Zn(s) |
-0.76 |
-25.76 |
-12.88 |
|
13 |
Ni+2 +
2e- = Ni(s) |
-0.24 |
-7.98 |
-3.99 |
|
14 |
Pb+2 +
2e- = Pb(s) |
-0.13 |
-4.27 |
-2.14 |
|
15 |
Cu+2 +
2e- = Cu(s) |
+0.34 |
11.48 |
5.74 |
|
16 |
Hg2+2 +
2e- = 2Hg(l) |
+0.91 |
30.79 |
15.40 |
|
17 |
Fe+2 +
2e- = Fe(s) |
-0.44 |
-14.9 |
-7.45 |
Properties of Selected Elements
|
Element |
Symbol |
Atomic # |
Atomic Wt. |
|
Electronegativity |
|
|
Aluminum |
Al |
13 |
26.98 |
3 |
1.47 |
|
|
Bromine |
Br |
35 |
79.904 |
1,3,5,7 |
2.74 |
|
|
Calcium |
Ca |
20 |
40.08 |
2 |
1.04 |
|
|
Carbon |
C |
6 |
12.01 |
2,4 |
2.50 |
|
|
Chlorine |
Cl |
17 |
35.453 |
1,3,5,7 |
2.83 |
|
|
Copper |
Cu |
29 |
63.54 |
1,2 |
1.75 |
|
|
Hydrogen |
H |
1 |
1.01 |
1 |
2.20 |
|
|
Magnesium |
Mg |
12 |
24.31 |
2 |
1.23 |
|
|
Manganese |
Mn |
25 |
54.94 |
2,3,4,6,7 |
1.60 |
|
|
Nitrogen |
N |
7 |
14.0047 |
3,5 |
3.07 |
|
|
Oxygen |
O |
8 |
16.00 |
2 |
3.50 |
|
|
Potassium |
K |
19 |
39.10 |
1 |
0.91 |
|
|
Sodium |
Na |
11 |
22.99 |
1 |
1.01 |
|
|
Strontium |
Sr |
38 |
87.62 |
2 |
0.99 |
|
|
Sulfur |
S |
16 |
32.06 |
2,4,6 |
2.44 |
|
|
Zinc |
Zn |
30 |
65.39 |
2 |
1.65 |
|
Selected Acidity Constants
(Aqueous Solution,
25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
Perchloric acid |
HClO4 = H+ + ClO4- |
-7 |
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
Sulfuric acid |
H2SO4= H+ + HSO4- |
-3 |
|
Nitric acid |
HNO3 = H+ + NO3- |
0 |
|
Bisulfate ion |
HSO4- = H+ +
SO4-2 |
2 |
|
Phosphoric acid |
H3PO4 = H+ + H2PO4- |
2.15 |
|
o-Phthalic acid |
C6H4(COOH)2 = H+ + C6H4(COOH)COO- |
2.89 |
|
p-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.48 |
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
Aluminum ion |
Al(H2O)6+3
= H+ + Al(OH)(H2O)5+2 |
4.8 |
|
Carbonic acid |
H2CO3 = H+ + HCO3- |
6.35 |
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02 |
|
Dihydrogen phosphate |
H2PO4- = H+ + HPO4-2 |
7.2 |
|
Hypochlorous acid |
HOCl = H+
+ OCl- |
7.5 |
|
Hypobromous acid |
HOBr = H+
+ OBr- |
8.71 |
|
Ammonium ion |
NH4+ = H+ + NH3 |
9.24 |
|
Bicarbonate ion |
HCO3- = H+ + CO3-2 |
10.33 |
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |