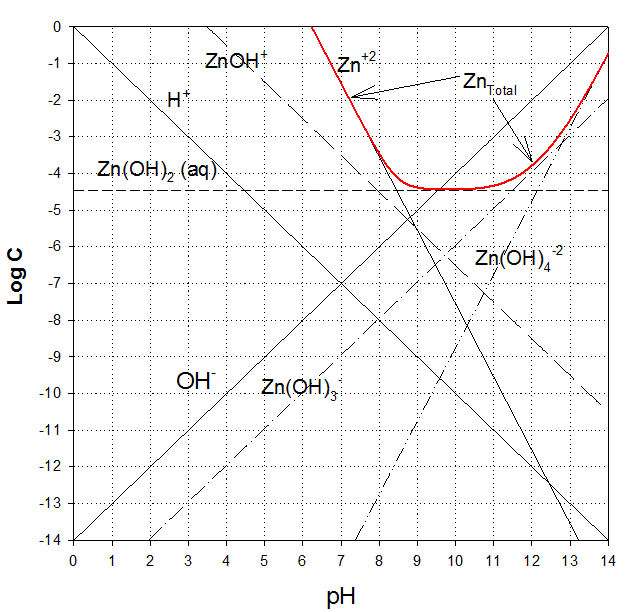|
CEE 680 |
|
18 December 2007 |
FINAL EXAM
Closed book, three pages of notes allowed.
Answer Question A and either B or C. Please state any additional assumptions you made, and show all work. If you don’t have time to complete a section, please describe how you would solve the problem (without using a computer program such as MINEQL).
Part I: Answer Question A
A. Solubility & Predominance. (75%)
Zinc carbonate (ZnCO3(s)) and Zinc Hydroxide (Zn(OH)2 (s)) are two important solid phases that may control zinc solubility in water[1]. In the attached pages is a detailed solution leading to a zinc hydroxide solubility diagram[2]. Please use this to help solve the following problems.
1. Prepare a solubility diagram (log C vs pH) for a water that is potentially in equilibrium with zinc hydroxide and zinc carbonate. Assume the water has 10-3 M total carbonates. Show all soluble species along with the ZnT line and indicate where precipitation will occur and the type of precipitate. Please feel free to use any of the hydroxide calculations in developing the answer to part 1 or part 2. It could save you some time.
2. Prepare a predominance diagram, showing the precipitates and major soluble species (in areas where there are no precipitates). As would be typical for a problem of this type, make pH the x-axis, and log total carbonate (CO3T), the y-axis. Assume a total soluble zinc concentration of 10-3 M (1 mM). Again you may find that using the solubility diagram provided or the one you did for part 1 can help in directing your work for part 2.
|
Equilibrium[3] |
Log K |
|
Zn |
-5.04 |
|
Zn(OH)2o = ZnOH+ + |
-6.06 |
|
Zn(OH)3-1 = Zn(OH)2o
+ |
-2.50 |
|
Zn(OH)4-2 = Zn(OH)3-1
+ |
-1.20 |
|
Zn(OH)2 (s) = Zn+2 + 2OH- |
-15.55 |
|
ZnCO3 (s) = Zn+2 + CO3-2 |
-10.26 |
Part II:. Answer either B or C
B. Redox (25%)
Galvanized pipe is still quite common in home plumbing in the US and Europe. The zinc coating on these pipe materials can corrode and undergo oxidation from the zero-valent metal to the soluble divalent aqueous zinc.
Residual active chlorine (hypochlorous acid) is a powerful oxidant. It can readily cause the oxidation of zinc metal to the divalent cation. However, in many drinking water systems, chlorine is not used as a disinfectant (e.g., groundwater systems) or it becomes substantially dissipated. The major residual oxidant is dissolved oxygen. After reaction, xxygen’s final reduced form is usually hydroxide and water. You have been asked to comment on the possibility that zinc can be oxidized by oxygen, and whether oxidized zinc can affect other metals. Specifically, your tasks are:
1. Write a balanced equation for the oxidation of metallic zinc to Zn+2 by dissolved oxygen
2. Determine the stoichiometry of this reaction (e.g., mg-O2/mg-Zn).
3. Determine the equilibrium constant (K) for this reaction and comment on what thermodynamics tells you about whether this is a favorable reaction.
4. Make a similar determination about the abililty of oxidized zinc (Zn+2 ) to oxidize metallic iron to Fe+2.
C. Redox True/False (25%) Mark each one of
the following statements with either a "T" or an "F"
a. ______ Lead is toxic partly because it has the same redox potential as calcium .
b. ______ As metals become more oxidized, they tend to be more prone to hydrolysis
c. ______ Most Redox reactions essentially result in complete conversion to products or no conversion at all when at equilibrium
d. ______ Phosphate helps control corrosion by chemically reducing certain oxidized metal species
e. ______ Environmental systems are far more likely to be at redox (e.g., electron) equilibrium than at acid/base (e.g., proton) equilibrium.
f. ______ Oxidation state is almost always changes with ligand number.
g. ______ The oxidation state of phosphorus in the environment is almost always +V.
h. ______ As pe increases, so does the electron activity
i. ______ The easiest way to measure half cell potentials is with a standard hydrogen electrode.
j. ______ For the ferrous/ferric redox couple, the value of ao plus a1 must always equal one.
Some important equilibrium constants:
|
Equilibria |
Log K |
|
Mg(OH)2 (s) = Mg+2 + 2OH- |
-11.6 |
|
Fe+3 + H2O = FeOH+2 + H+ |
-2.19 |
|
Mg+2 + H2O = MgOH+ + H+ |
-11.44 |
|
MgCO3 (s) = Mg+2 + CO3-2 |
-7.5 |
|
CaCO3(s) = Ca+2 + CO3-2 |
-8.34 |
|
Ca(OH2)(s) = Ca+2 + 2OH- |
-5.19 |
|
CaSO4.2H2O(s)
= Ca+2 + SO4-2 + 2H2O |
-4.62 |
|
CaOH+ = Ca+2 + |
-1.15 |
|
AlOH+2 = Al+3 + |
-9.01 |
|
CdOH+ = Cd+2 + |
-3.92 |
|
Co |
-4.80 |
|
Cu |
-6.00 |
|
FeOH+ = Fe+2 + |
-4.50 |
|
Hg |
-10.60 |
|
NiOH+ = Ni+2 + |
-4.14 |
|
Pb |
-6.29 |
|
Zn |
-5.04 |
Some
important half-cell reactions
|
Equ# |
Half
Cell Reaction |
DEo (Volts) |
|
1 |
O2(g) + 4H+ + 4e- = 2H2O |
+1.23 |
|
2 |
Mn+3 +
e- = Mn+2 |
+1.51 |
|
3 |
Mn+4 +
e- = Mn+3 |
+1.65 |
|
4 |
MnO4- + 8H+ + 5e- = Mn+2 + 4H2O |
+1.49 |
|
5 |
Fe+3 +
e- = Fe+2 |
+0.77 |
|
6 |
Cu+2 + e- = Cu+ |
+0.16 |
|
7 |
½HOBr + ½H+ + e- =
½Br- + ½H2O |
+1.33 |
|
8 |
O3 (g) + 2H+ + 2 e- = O2 (g) + H2O |
+2.07 |
|
9 |
Al+3 + 3e- = Al(s) |
-1.68 |
|
10 |
S(s) + 2H+ + 2e- = H2S (g) |
+0.17 |
|
11 |
½NH2Cl + H+ +e-
= ½Cl- + ½NH4+ |
+1.40 |
|
12 |
Zn+2 +
2e- = Zn(s) |
-0.76 |
|
13 |
Ni+2 +
2e- = Ni(s) |
-0.24 |
|
14 |
Pb+2 +
2e- = Pb(s) |
-0.13 |
|
15 |
Cu+2 +
2e- = Cu(s) |
+0.34 |
|
16 |
Hg2+2 +
2e- = 2Hg(l) |
+0.91 |
|
17 |
Fe+2 +
2e- = Fe(s) |
-0.44 |
Properties of Selected Elements
|
Element |
Symbol |
Atomic # |
Atomic Wt. |
|
Electronegativity |
|
|
Aluminum |
Al |
13 |
26.98 |
3 |
1.47 |
|
|
Bromine |
Br |
35 |
79.904 |
1,3,5,7 |
2.74 |
|
|
Calcium |
Ca |
20 |
40.08 |
2 |
1.04 |
|
|
Carbon |
C |
6 |
12.01 |
2,4 |
2.50 |
|
|
Chlorine |
Cl |
17 |
35.453 |
1,3,5,7 |
2.83 |
|
|
Copper |
Cu |
29 |
63.54 |
1,2 |
1.75 |
|
|
Hydrogen |
H |
1 |
1.01 |
1 |
2.20 |
|
|
Magnesium |
Mg |
12 |
24.31 |
2 |
1.23 |
|
|
Manganese |
Mn |
25 |
54.94 |
2,3,4,6,7 |
1.60 |
|
|
Nitrogen |
N |
7 |
14.0047 |
3,5 |
3.07 |
|
|
Oxygen |
O |
8 |
16.00 |
2 |
3.50 |
|
|
Potassium |
K |
19 |
39.10 |
1 |
0.91 |
|
|
Sodium |
Na |
11 |
22.99 |
1 |
1.01 |
|
|
Strontium |
Sr |
38 |
87.62 |
2 |
0.99 |
|
|
Sulfur |
S |
16 |
32.06 |
2,4,6 |
2.44 |
|
Selected Acidity Constants
(Aqueous Solution, 25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
Perchloric acid |
HClO4 = H+ +
ClO4- |
-7 |
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
Sulfuric acid |
H2SO4=
H+ + HSO4- |
-3 |
|
Nitric acid |
HNO3 = H+ +
NO3- |
0 |
|
Bisulfate ion |
HSO4-
= H+ + SO4-2 |
2 |
|
Phosphoric acid |
H3PO4 =
H+ + H2PO4- |
2.15 |
|
o-Phthalic acid |
C6H4(COOH)2
= H+ + C6H4(COOH)COO- |
2.89 |
|
p-Hydroxybenzoic acid |
C6H4(OH)COOH
= H+ + C6H4(OH)COO- |
4.48
|
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
Aluminum ion |
Al(H2O)6+3 = H+ + Al(OH)(H2O)5+2 |
4.8 |
|
Carbonic acid |
H2CO3 =
H+ + HCO3- |
6.35
|
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02
|
|
Dihydrogen phosphate |
H2PO4-
= H+ + HPO4-2 |
7.2 |
|
Hypochlorous acid |
HOCl = H+ + OCl- |
7.5 |
|
Hypobromous acid |
HOBr = H+ + OBr- |
8.71 |
|
Ammonium ion |
NH4+
= H+ + NH3 |
9.24 |
|
Bicarbonate ion |
HCO3-
= H+ + CO3-2 |
10.33 |
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |
Development of hydroxide solubility diagram.
a. Using the
equilibria for zinc hydroxide from above:
![]()
we get the following for the free aquo ion:
![]()
![]()
![]()
From this we use the hydroxide equilibria to get hydroxide species concentrations:
and for the monohydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
now for the dihydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
now for the trihydroxide:
![]()
![]()
![]()
![]()
![]()
![]()
now for the tetrahydroxide:
![]()
![]()
![]()
![]()
![]()
![]()