|
CEE 680 |
|
15 November 2007 |
SECOND EXAM
Closed book, two pages of notes allowed.
Answer all questions. Please state any additional assumptions you made, and show all work.
1. Carbonate System.
(50% for both parts) Two raw drinking waters are mixed as they enter the headworks of a water treatment plant. The two are characterized as follows:
|
Water |
Flow (MGD) |
Alkalinity (mg/L as CaCO3) |
pH |
|
#1 |
20 |
5 |
6.50 |
|
#2 |
10 |
350 |
8.85 |
A. What will the pH of the blended water be immediately after mixing?
B. What will the pH of the blended water be after it has reached equilibrium with the bulk atmosphere?
2. Complexation
(40%
for both parts) Aqueous
bromide forms strong complexes with many metals. The following two part problem concerns
complexes with silver.
A.
(20%)
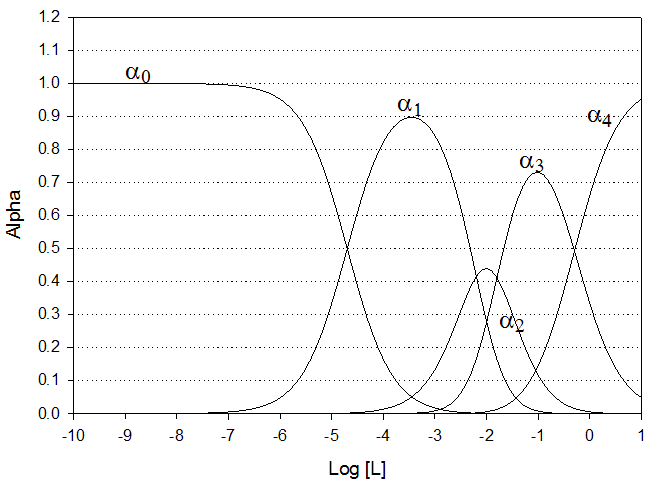
Attached is an accurate graph of alpha values (vs log[ Br-]) for the
Silver-bromide system (equilibria data shown below). Using this graph determine the
complete species composition when the total silver concentration is 20 mM and
the total bromide concentration is 10 mM.
Ignore the possible formation of any
other complexes other than those from Ag and Br; also ignore any possible
precipitation reactions.
B.
(20%)
Describe in qualitative terms the impacts of addition of a very powerful ligand
such as EDTA to this mixture. Estimate
in quantitative terms what the concentrations of each of the species would be
if the total EDTA added was 10 mM, and explain how you got these.
3. Multiple Choice.
(10%) Answer all 10 of the following questions. Indicate which of the options is the best choice.
1. Benjamin is:
a. the last name of your textbook’s author
b. the first name of your textbook’s author
c. the first name of the current US president
d. a unit of hardness
e. one of the halogens
2. When a solution spontaneously absorbs CO2 from the atmosphere it:
a. results in higher total carbonate
b. drops in pH
c. approaches equilibrium
d. all of the above
e. none of the above
3. Phosphate
a. is a hexadentate ligand
b. is a deadly poison
c. is insoluble
d. is the drug of choice for malaria
e. has been used as a “builder” in detergents
4. H2CO3*:
a. is composed mostly of aqueous CO2
b. is always conservative, even in open systems
c. is a republican
d. all of the above
e. complexes very strongly with sodium
5. Ion pairs:
a. are always charged
b. are larger than
c. are illegal in Montana
d. are outer-sphere complexes
6. The coordination number:
a. is usually 6 or less
b. is related to the charge on the central atom
c. is a function of the size of the ligand
d. all of the above
e. none fo the obove
7. The buffer intensity of the open carbonate system:
a. is independent of the alkalinity
b. is
c. is always higher than the pCO2
d. is at a minimum where the pH = pK1
e. is at a minimum where the pH = pK2
8. Detergent “builders” are used to:
a. help solubilize grease
b. complex trace metals
c. take hardness cations from the surfactants
d. elevate the acidity
e. reduce the caloric content
9. EDTA
a. stands for ethylene dinitro tetraacetic acid
b. is most commonly used as a pH buffer
c. is a higly potent carcinogen
d. is never used because no-one knows what it stands for
e. is a multi-dentate ligand
10. The
a. is a means of estimating alkalinity
b. describes the inverse proportionality of acidity to alkalinity
c. includes a number of books, such as The Chapman Report, and The Prize
d. provides a comprehensive description of ligand structure
e. follows the increase in ligand affinity from Mn(II) to Cu(II)
Selected Acidity Constants (Aqueous Solution, 25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
|
Perchloric acid |
HClO4 = H+ + ClO4- |
-7 STRONG |
|
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
|
Sulfuric acid |
H2SO4= H+ + HSO4- |
-3 (&2) ACIDS |
|
|
Nitric acid |
HNO3 = H+ + NO3- |
-0 |
|
|
Hydronium ion |
H3O+ = H+ + H2O |
0 |
|
|
Trichloroacetic acid |
CCl3COOH = H+ + CCl3COO- |
0.70 |
|
|
Iodic acid |
HIO3 = H+ + IO3- |
0.8 |
|
|
Bisulfate ion |
HSO4- = H+ +
SO4-2 |
2 |
|
|
Phosphoric acid |
H3PO4 = H+ + H2PO4- |
2.15 (&7.2,12.3) |
|
|
Citric acid |
C3H5O(COOH)3=
H+ + C3H5O(COOH)2COO- |
3.14 (&4.77,6.4) |
|
|
Hydrofluoric acid |
HF = H+
+ F- |
3.2 |
|
|
m-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.06 (&9.92) |
|
|
p-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.48 (&9.32) |
|
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
|
Propionic acid |
C2H5COOH = H+ + C2H5COO- |
4.87 |
|
|
Carbonic acid |
H2CO3 = H+ + HCO3- |
6.35 (&10.33) |
|
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02 (&13.9) |
|
|
Dihydrogen phosphate |
H2PO4- = H+ + HPO4-2 |
7.2 |
|
|
Hypochlorous acid |
HOCl = H+
+ OCl- |
7.5 |
|
|
Boric acid |
B(OH)3 + H2O = H+ + B(OH)4- |
9.2 (&12.7,13.8) |
|
|
Ammonium ion |
NH4+ = H+ + NH3 |
9.24 |
|
|
Hydrocyanic acid |
HCN = H+
+ CN- |
9.3 |
|
|
p-Hydroxybenzoic acid |
C6H4(OH)COO- = H+ + C6H4(O)COO-2 |
9.32 |
|
|
Phenol |
C6H5OH = H+ + C6H5O- |
9.9 |
|
|
m-Hydroxybenzoic acid |
C6H4(OH)COO- = H+ + C6H4(O)COO-2 |
9.92 |
|
|
Bicarbonate ion |
HCO3- = H+ + CO3-2 |
10.33 |
|
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |
|
|
Bisulfide ion |
HS- = H+ + S-2 |
13.9 |
|
|
Water |
H2O = H+ + |
14.00 |
|
|
Methane |
CH4 = H+ + CH3- |
34 |
|