CEE
680
|
17 October 2007 |
FIRST EXAM
Closed book, one page of
notes allowed.
Answer
all questions. Please state any
additional assumptions you made, and show all work. You are welcome to use a graphical method of
solution if it is appropriate.
Miscellaneous Information:
R = 1.987 cal/mole°K = 8.314 J/mole°K
Absolute zero = -273.15°C
1 joule = 0.239 calories
1 parsec = 19,173,511,600,000 miles
1. (50%) What is the pH of a 10-2 M solution of Sodium Nitrite (NaNO2) to which you have added 5 x 10-3 M Nitric Acid (HNO3)? Calculate this for each of the three conditions below (obviously the ionic strength will never be zero for this solution, but let’s assume that ideal case for part “a” and “b”, anyway).
a. 25°C, I = 0
b. 1°C, I = 0
c. 25°C, I = 0.10
Preferred Approach
· recognize that this is a simple base problem
· then adopt an appropriate set of assumptions, and solve for [H+]
· finally correct pKa (and pKw) for temperature, ionic strength, and repeat
· check assumptions
a. 25°C, I = 0
First, recognize that nitric is a strong acid. Also, the addition of 5 mM
of nitric acid to 10 mM of sodium nitrite will result
in the equivalent of a 5 mM solution of nitrous acid
and a 5 mM solution of nitrite.
Next, make the usual buffer assumptions:
Ø
both H+ and
And then use the buffer equation:
![]()
So, considering that we’re dealing with the two adjacent
nitrite species HNO2 and NO2-, we have a
system centered on the Ka for the nitrite system.
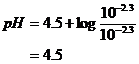
pH = 4.5
Check Assumptions:
[NO2-] >> [OH-]
10-2.3 >> 10-9.5 YES!!
[Na+]-[NO3-] >> [H+]
10-2.3 >>
10-4.5 YES!!
b. 1°C, I = 0
determine enthalpy change for the
reaction:
HNO2- = H+
+ NO2-
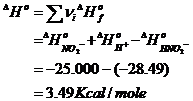
then re-estimate Ka
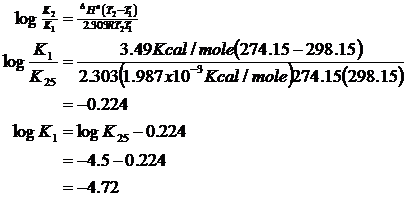
Or:
K1 = 1.89 x 10-5
and now:
Make the usual buffer
assumptions:
Ø
both H+ and
And then use the buffer
equation:
![]()
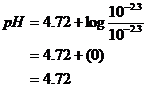
pH = 4.72
Check Assumptions:
[NO2-] >> [OH-]
10-2.3 >> 10-9.28 YES!!
[Na+]-[NO3-] >> [H+]
10-2.3 >>
10-4.72 YES!!
Now, strictly speaking, you would
also need to adjust Kw for the higher temperature
as well.
c. 25°C, I = 0.10
determine activity coefficients for
the species in the reaction:
H2PO4- = H+
+ HPO4-2
And for
H2O = H+ +
For this level,
use the simple Davies equation for the charged species, and assume no change in
the activity of the uncharged species:
![]()
So for the
singly-charged species:
![]()
![]()
then re-estimate Ka
and Kw under this condition, i.e., the
conditional K’s
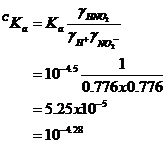
and now:
Make the same buffer
assumptions:
Ø
both H+ and
And then use the buffer
equation:
![]()
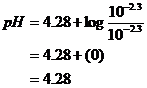
pH = 4.28
Check Assumptions:
[NO2-] >> [OH-]
10-2.3 >> 10-9.72
YES!!
[Na+]-[NO3-] >> [H+]
10-2.3 >>
10-4.28 YES!!
2. (40%) What is the complete composition of a 1-liter volume of water to which you have added 10-2 M of ammonium acetate (NH4CH3CO2)? Approximate values (± 0.2 log units) will suffice.
Approach
· prepare a logC vs pH diagram for ammonia system (CT=0.01 M) and the acetic acid system (CT = 0.01M) superimposed over it.
· write the PBE and find a solution
· read off concentrations from the graph
This is a good problem for
the graphical solution (no acid/base conjugates added, nor any strong acids or
bases). The first task is then to
prepare the species lines on our usual log C vs pH
axes (see below)
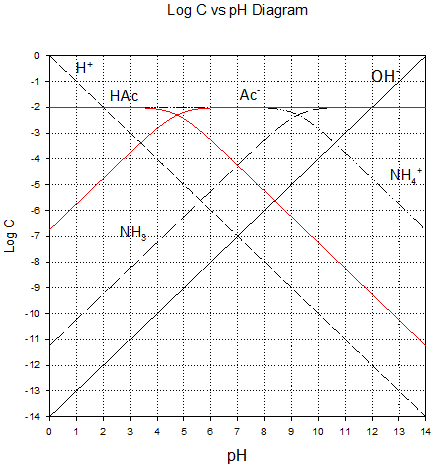
Recall that we’re adding ammonium cation (NH4+)
and the deprotonated acetate (I’ll call this: Ac-). These are simple solutions of two unrelated
acids/bases. Therefore we don’t have any
acid/conjugate base mixtures, nor do we have an acids or bases that have been
partly titrated with a strong acid or base.
This means we are free to use the PBE, and in fact, should use the PBE
(an ENE won’t give us a “clean” or identifiable intersection).
Thus, the PBE is:
[HAc]
+ [H+] = [OH-] + [NH3]
And if we presume that H+
and
[HAc]
= [NH3]
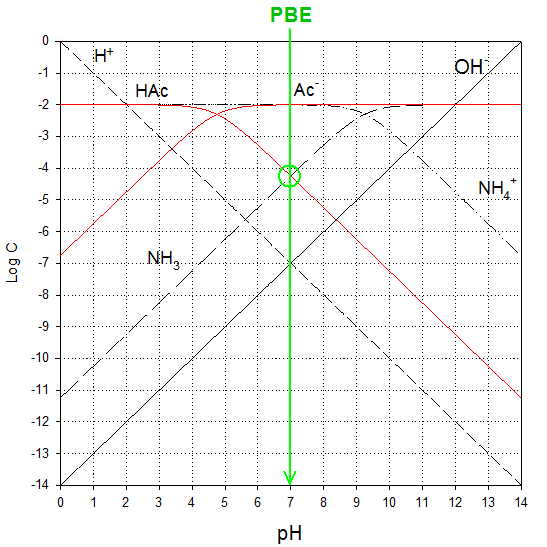
|
pH ≈ 7.0 |
[H+] ≈ 1 x 10-7 |
|
log [HAc] ≈ -4.3 |
[HAc] ≈ 5.0 x 10-5 |
|
log [Ac-]
≈ -2.0 |
[Ac-]
≈ 1 x 10-2 |
|
|
|
|
log [NH4+]
≈ -2.0 |
[NH4+]
≈ 1 x 10-2 |
|
log [NH3]
≈ -4.3 |
[NH3]
≈ 5.0 x 10-5 |
|
log [OH-]
≈ -7.0 |
[OH-]
≈ 1 x 10-7 |
Check assumptions:
[HAc]
>> [H+]
10-4.3 >>
10-7.0, YES
[NH3] >>
[OH-]
10-4.3 >>
10-7.0, again YES
3. (10%) True/False. Mark each one of the following statements with either a "T" or an "F".
a. ___ T_ Solutions of pure compounds exhibit
their highest buffer intensities at pHs near the compound’s pKa .
b. ___ F_ Solutions of hydrochloric acid are
always lower in pH than solutions of acetic acid.
c. ___ T_ A 10-2 F solution of HCl has a pH of about 2.
d. ___ F_ Strong acids will always have strong
conjugate bases.
e. ___ T_ The sum of alkalinity and acidity equals
twice the total inorganic carbon.
f. ___ T_ Dihydrogen
phosphate (H2PO4-) is an amphoteric
substance.
g. ___ F_ The buffer intensity of a solution is
the inverse of the alkalinity.
h. ___ F_ Positive DH values indicate that the
reaction is not spontaneous
i. ___ T_ The standard assumption used for
calculating the pH of an acidic solution is that the [OH-] is
negligible.
j. ___ F_ For a diprotic
acid, the value of ao plus a1 must always equal one.
Selected
Acidity Constants (Aqueous
Solution, 25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
|
Perchloric acid |
HClO4 = H+ +
ClO4- |
-7 STRONG |
|
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
|
Sulfuric acid |
H2SO4= H+ + HSO4- |
-3 (&2) ACIDS |
|
|
Nitric acid |
HNO3 = H+ + NO3- |
-0 |
|
|
Hydronium ion |
H3O+ = H+ + H2O |
0 |
|
|
Trichloroacetic acid |
CCl3COOH = H+ + CCl3COO- |
0.70 |
|
|
Iodic acid |
HIO3 = H+ + IO3- |
0.8 |
|
|
Bisulfate ion |
HSO4- = H+ +
SO4-2 |
2 |
|
|
Phosphoric acid |
H3PO4 = H+ + H2PO4- |
2.15 (&7.2,12.3) |
|
|
o-Phthalic acid |
C6H4(COOH)2 = H+ + C6H4(COOH)COO- |
2.89 (&5.51) |
|
|
Citric acid |
C3H5O(COOH)3=
H+ + C3H5O(COOH)2COO- |
3.14 (&4.77,6.4) |
|
|
Hydrofluoric acid |
HF = H+
+ F- |
3.2 |
|
|
Aspartic acid |
C2H6N(COOH)2= H+ + C2H6N(COOH)COO- |
3.86 (&9.82) |
|
|
m-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.06 (&9.92) |
|
|
p-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.48 (&9.32) |
|
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
|
Propionic acid |
C2H5COOH = H+ + C2H5COO- |
4.87 |
|
|
Carbonic acid |
H2CO3 = H+ + HCO3- |
6.35 (&10.33) |
|
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02 (&13.9) |
|
|
Dihydrogen phosphate |
H2PO4- = H+ + HPO4-2 |
7.2 |
|
|
Hypochlorous acid |
HOCl = H+ + OCl- |
7.5 |
|
|
Boric acid |
B(OH)3 + H2O = H+ + B(OH)4- |
9.2 (&12.7,13.8) |
|
|
Ammonium ion |
NH4+ = H+ + NH3 |
9.24 |
|
|
Hydrocyanic acid |
HCN = H+
+ CN- |
9.3 |
|
|
p-Hydroxybenzoic acid |
C6H4(OH)COO- = H+ + C6H4(O)COO-2 |
9.32 |
|
|
Phenol |
C6H5OH = H+ + C6H5O- |
9.9 |
|
|
m-Hydroxybenzoic acid |
C6H4(OH)COO- = H+ + C6H4(O)COO-2 |
9.92 |
|
|
Bicarbonate ion |
HCO3- = H+ + CO3-2 |
10.33 |
|
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |
|
|
Bisulfide ion |
HS- = H+ + S-2 |
13.9 |
|
|
Water |
H2O = H+ + |
14.00 |
|
|
Ammonia |
NH3 = H+ + NH2- |
23 |
|
|
Methane |
CH4 = H+ + CH3- |
34 |
|
|
Species |
kcal/mole |
kcal/mole |
|
Ca+2(aq) |
‑129.77 |
‑132.18 |
|
CaC03(s), calcite |
‑288.45 |
‑269.78 |
|
CaO (s) |
‑151.9 |
‑144.4 |
|
C(s), graphite |
0 |
0 |
|
CO2(g) |
‑94.05 |
‑94.26 |
|
CO2(aq) |
‑98.69 |
‑92.31 |
|
CH4 (g) |
‑17.889 |
‑12.140 |
|
H2CO3 (aq) |
‑167.0 |
‑149.00 |
|
HCO3- (aq) |
‑165.18 |
‑140.31 |
|
CO3-2 (aq) |
‑161.63 |
‑126.22 |
|
CH3COO-, acetate |
‑116.84 |
‑89.0 |
|
H+ (aq) |
0 |
0 |
|
H2 (g) |
0 |
0 |
|
HF (aq) |
-77.23 |
-71.63 |
|
F- (aq) |
-80.15 |
-67.28 |
|
Fe+2 (aq) |
‑21.0 |
‑20.30 |
|
Fe+3 (aq) |
‑11.4 |
‑2.52 |
|
NO2- (aq) |
‑25.00 |
‑8.89 |
|
NO3- (aq) |
‑49.372 |
‑26.43 |
|
NH3 (g) |
‑11.04 |
‑3.976 |
|
NH3 (aq) |
‑19.32 |
‑6.37 |
|
NH4+ (aq) |
‑31.74 |
‑19.00 |
|
HNO2 (aq) |
‑28.49 |
‑10.27 |
|
HNO3 (aq) |
‑49.372 |
‑26.41 |
|
O2 (aq) |
‑3.9 |
3.93 |
|
O2 (g) |
0 |
0 |
|
|
‑54.957 |
‑37.595 |
|
H2O (g) |
‑57.7979 |
‑54.6357 |
|
H2O (l) |
‑68.3174 |
‑56.690 |
|
PO4-3 (aq) |
-305.30 |
-243.50 |
|
HPO4-2 (aq) |
-308.81 |
-260.34 |
|
H2PO4- (aq) |
-309.82 |
-270.17 |
|
H3PO4 (aq) |
-307.90 |
-273.08 |
|
SO4-2 |
‑216.90 |
‑177.34 |
|
HS- (aq) |
‑4.22 |
3.01 |
|
H2S(g) |
‑4.815 |
‑7.892 |
|
H2S(aq) |
‑9.4 |
‑6.54 |