CEE
680
|
12 October 2006 |
FIRST EXAM
Closed book, one page of
notes allowed.
Answer
all questions. Please state any
additional assumptions you made, and show all work. You are welcome to use a graphical method of
solution if it is appropriate.
Miscellaneous Information:
R = 1.987 cal/mole°K = 8.314 J/mole°K
Absolute zero = -273.15°C
1 joule = 0.239 calories
1 parsec = 19,173,511,600,000 miles
1. (50%) What is the pH of a 10-3 M solution of Sodium Dihydrogen Phosphate (NaH2PO4) and 10-2.7 M Sodium Monohydrogen Phosphate (Na2HPO4)? Calculate this for each of the three conditions below.
a. 25°C, I = 0
b. 100°C, I = 0
c. 25°C, I = 0.25
Preferred Approach
· recognize that this is a simple base problem
· then adopt an appropriate set of assumptions, and solve for [H+]
· finally correct pKa (and pKw) for temperature, ionic strength, and repeat
· check assumptions
a. 25°C, I = 0
Make the usual buffer
assumptions:
Ø
both H+ and
And then use the buffer
equation:
![]()
So, considering that we’re dealing with the two amphoteric phosphate species H2PO4-
and HPO4-2, we have a system centered on the second Ka
for the phosphate system.
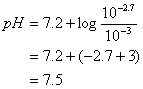
pH = 7.5
Check Assumptions:
[H2PO4-] + 2[HPO4-2]
>> [OH-]
10-3 + 2x10-2.7 >> 10-6.5 YES!!
[Na+] >> [H+]
2x10-3 + 10-2.7
>> 10-7.5
YES!!
b. 100°C, I = 0
determine enthalpy change for the
reaction:
H2PO4- =
H+ + HPO4-2
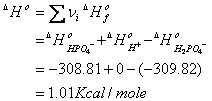
then re-estimate Ka
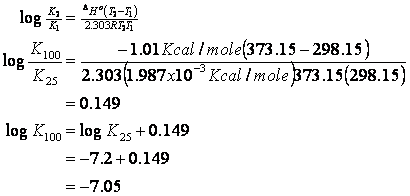
Or:
K100 = 8.89 x 10-8
and now:
Make the usual buffer
assumptions:
Ø
both H+ and
And then use the buffer
equation:
![]()
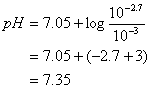
pH = 7.35
Check Assumptions:
[H2PO4-]
+ 2[HPO4-2] >> [
10-3 + 2x10-2.7
>> 10-6.65
YES!!
[Na+] >> [H+]
2x10-3 + 10-2.7
>> 10-7.35
YES!!
Now, strictly speaking, you would also
need to adjust Kw for the higher
temperature as well.
c. 25°C, I = 0.25
determine activity coefficients for
the species in the reaction:
H2PO4- =
H+ + HPO4-2
And for
H2O = H+ +
For this level, use the
simple Davies equation for the charged species, and assume no change in the
activity of theuncharged species:
![]()
So for the
singly-charged species:
![]()
![]()
And for the
doubly-charged species:
![]()
![]()
then re-estimate Ka
and Kw under this condition, i.e., the
conditional K’s

and:

and now:
Make the same buffer
assumptions:
Ø
both H+ and
And then use the buffer
equation:
![]()
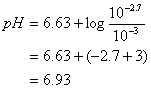
pH = 6.93
Check Assumptions:
[H2PO4-]
+ 2[HPO4-2] >> [
10-3 + 2x10-2.7
>> 10-6.79
YES!!
[Na+] >> [H+]
2x10-3 + 10-2.7
>> 10-6.93
YES!!
2. (40%) What is the complete composition of a 1-liter volume of water to which you have added 10-1.5 M of ammonium nitrate (NH4NO3) and 10-2.5 M of the mono-sodium salt of p-Hydroxybenzoic Acid? Approximate values (± 0.2 log units) will suffice.
Approach
· prepare a logC vs pH diagram for carbonate system (CT=0.001 M) and the acetic acid system (CT = 0.01M) superimposed over it.
· write the PBE and find a solution
· read off concentrations from the graph
This is a good problem for
the graphical solution (no acid/base conjugates added, nor any strong acids or
bases). The first task is then to
prepare the species lines on our usual log C vs pH
axes (see below)
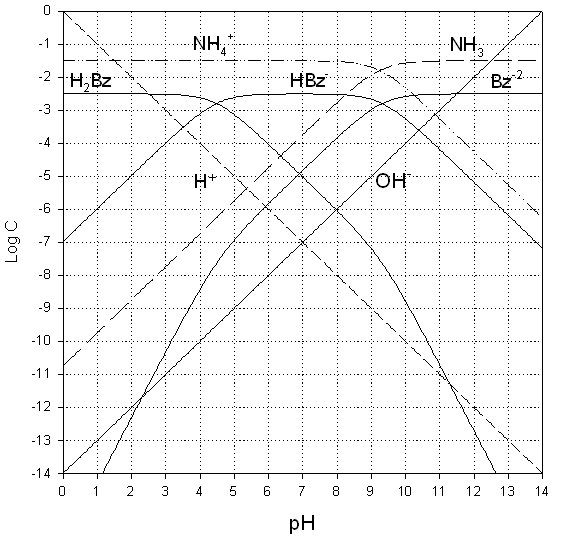
Recall that we’re adding ammonium cation (NH4+)
and the singly-deprotonated hydroxyl-benzoate (I’ll
call this: HBz-). These are simple solutions of two unrelated
acids/bases. Therefore we don’t have any
acid/conjugate base mixtures, nor do we have an acids or bases that have been
partly titrated with a strong acid or base.
This means we are free to use the PBE, and in fact, should use the PBE
(an ENE won’t give us a “clean” or identifiable intersection).
Thus, the PBE is:
[H2Bz] + [H+]
= [
And if we presume that H+
and
[H2Bz] = [Bz-2]
+ [NH3]
from the graph above, its easy
to see that [NH3] is always
above [Bz-2], so that the PBE solution lies at:
[H2Bz] = [NH3]
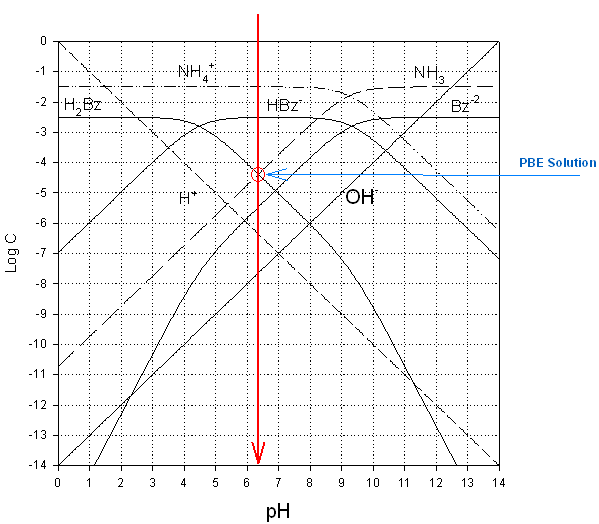
|
pH ≈
6.3 |
[H+] ≈ 5.0 x 10-7 |
|
log [H2Bz]
≈ -4.4 |
[H2CO3]
≈ 4.0 x 10-5 |
|
log [HBz-] ≈ -2.5 |
[HCO3-]
≈ 3.2 x 10-3 |
|
log [Bz-2]
≈ -5.4 |
[CO3-2]
≈ 4.0 x 10-6 |
|
log [NH4+]
≈ -1.5 |
[HAc] ≈ 3.2 x 10-2 |
|
log [NH3]
≈ -4.4 |
[Ac-]
≈ 4.0 x 10-5 |
|
log [ |
[ |
Check assumptions:
[H2Bz] >>
[H+]
10-4.4 >>
10-6.3, YES
[NH3] >> [
10-4.4 >>
10-7.7 + 10-5.4, closer, but still YES
3. (10%) True/False. Mark each one of the following statements with either a "T" or an "F".
a. F____ An increase in ionic strength will affect hydrogen ion
activity, but not hydrogen ion concentration.
b. T____ Titration curves (pH vs g or f)
always exhibit a local minimum slope at a pH equal to the pKa
of the acid/base being titrated.
c. T____ Electrons do not usually exist in a
d. F____ Strong acids will always cause the pH to drop below 2.
e. T____ Alkalinity is a measure of acid neutralizing capacity.
f. F____ Sodium Acetate is an amphoteric
substance.
g. F____ The buffer intensity of a solution of a pure acid is at
its lowest at a pH near the pKa of the acid.
h. F____ Positive DH values indicate that the
reaction is exothermic
i. F____ The standard assumption used for calculating the pH of an
acidic solution is that the [H+] is negligible.
j. F____ For a triprotic acid, the value
of ao plus a1 must always equal one.
Selected
Acidity Constants (Aqueous
Solution, 25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
|
Perchloric acid |
HClO4 = H+ +
ClO4- |
-7 STRONG |
|
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
|
Sulfuric acid |
H2SO4= H+ + HSO4- |
-3 (&2) ACIDS |
|
|
Nitric acid |
HNO3 = H+ + NO3- |
-0 |
|
|
Hydronium ion |
H3O+ = H+ + H2O |
0 |
|
|
Trichloroacetic acid |
CCl3COOH = H+ + CCl3COO- |
0.70 |
|
|
Iodic acid |
HIO3 = H+ + IO3- |
0.8 |
|
|
Bisulfate ion |
HSO4- = H+ +
SO4-2 |
2 |
|
|
Phosphoric acid |
H3PO4 = H+ + H2PO4- |
2.15 (&7.2,12.3) |
|
|
o-Phthalic acid |
C6H4(COOH)2 = H+ + C6H4(COOH)COO- |
2.89 (&5.51) |
|
|
Citric acid |
C3H5O(COOH)3=
H+ + C3H5O(COOH)2COO- |
3.14 (&4.77,6.4) |
|
|
Hydrofluoric acid |
HF = H+
+ F- |
3.2 |
|
|
Aspartic acid |
C2H6N(COOH)2= H+ + C2H6N(COOH)COO- |
3.86 (&9.82) |
|
|
m-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.06 (&9.92) |
|
|
p-Hydroxybenzoic acid |
C6H4(OH)COOH = H+ + C6H4(OH)COO- |
4.48 (&9.32) |
|
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
|
Propionic acid |
C2H5COOH = H+ + C2H5COO- |
4.87 |
|
|
Carbonic acid |
H2CO3 = H+ + HCO3- |
6.35 (&10.33) |
|
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02 (&13.9) |
|
|
Dihydrogen phosphate |
H2PO4- = H+ + HPO4-2 |
7.2 |
|
|
Hypochlorous acid |
HOCl = H+ + OCl- |
7.5 |
|
|
Boric acid |
B(OH)3 + H2O = H+ + B(OH)4- |
9.2 (&12.7,13.8) |
|
|
Ammonium ion |
NH4+ = H+ + NH3 |
9.24 |
|
|
Hydrocyanic acid |
HCN = H+
+ CN- |
9.3 |
|
|
p-Hydroxybenzoic acid |
C6H4(OH)COO- = H+ + C6H4(O)COO-2 |
9.32 |
|
|
Phenol |
C6H5OH = H+ + C6H5O- |
9.9 |
|
|
m-Hydroxybenzoic acid |
C6H4(OH)COO- = H+ + C6H4(O)COO-2 |
9.92 |
|
|
Bicarbonate ion |
HCO3- = H+ + CO3-2 |
10.33 |
|
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |
|
|
Bisulfide ion |
HS- = H+ + S-2 |
13.9 |
|
|
Water |
H2O = H+ + |
14.00 |
|
|
Ammonia |
NH3 = H+ + NH2- |
23 |
|
|
Methane |
CH4 = H+ + CH3- |
34 |
|
|
Species |
kcal/mole |
kcal/mole |
|
Ca+2(aq) |
‑129.77 |
‑132.18 |
|
CaC03(s),
calcite |
‑288.45 |
‑269.78 |
|
CaO (s) |
‑151.9 |
‑144.4 |
|
C(s), graphite |
0 |
0 |
|
CO2(g) |
‑94.05 |
‑94.26 |
|
CO2(aq) |
‑98.69 |
‑92.31 |
|
CH4 (g) |
‑17.889 |
‑12.140 |
|
H2CO3
(aq) |
‑167.0 |
‑149.00 |
|
HCO3-
(aq) |
‑165.18 |
‑140.31 |
|
CO3-2
(aq) |
‑161.63 |
‑126.22 |
|
CH3COO-,
acetate |
‑116.84 |
‑89.0 |
|
H+ (aq) |
0 |
0 |
|
H2 (g) |
0 |
0 |
|
HF (aq) |
-77.23 |
-71.63 |
|
F- (aq) |
-80.15 |
-67.28 |
|
Fe+2 (aq) |
‑21.0 |
‑20.30 |
|
Fe+3 (aq) |
‑11.4 |
‑2.52 |
|
NO3-
(aq) |
‑49.372 |
‑26.43 |
|
NH3
(g) |
‑11.04 |
‑3.976 |
|
NH3
(aq) |
‑19.32 |
‑6.37 |
|
NH4+
(aq) |
‑31.74 |
‑19.00 |
|
HNO3
(aq) |
‑49.372 |
‑26.41 |
|
O2
(aq) |
‑3.9 |
3.93 |
|
O2 (g) |
0 |
0 |
|
|
‑54.957 |
‑37.595 |
|
H2O (g) |
‑57.7979 |
‑54.6357 |
|
H2O (l) |
‑68.3174 |
‑56.690 |
|
PO4-3
(aq) |
-305.30 |
-243.50 |
|
HPO4-2
(aq) |
-308.81 |
-260.34 |
|
H2PO4-
(aq) |
-309.82 |
-270.17 |
|
H3PO4
(aq) |
-307.90 |
-273.08 |
|
SO4-2 |
‑216.90 |
‑177.34 |
|
HS- (aq) |
‑4.22 |
3.01 |
|
H2S(g) |
‑4.815 |
‑7.892 |
|
H2S(aq) |
‑9.4 |
‑6.54 |