|
CEE 670 |
Fall 2010 |
Kinetics Homework #2
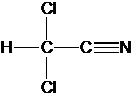
1. The data below are for the
decomposition of Dichloroacetonitrile (DCAN) at pH 7, and 20 C. One column is for and ionic strength of 0.01
M (mostly NaCl), and the other for 0.03 M.
Analyze these data and make any conclusions you can regarding the nature
of the hydrolysis reaction of DCAN.
|
|
DCAN
Concentration (µg/L) |
|
|
Time
(hours) |
I
= 0.01 M |
I
= 0.03 M |
|
0.00 |
44.49 |
44.74 |
|
0.75 |
41.10 |
42.29 |
|
1.50 |
43.73 |
41.64 |
|
2.50 |
38.84 |
37.87 |
|
3.50 |
38.27 |
38.45 |
|
6.50 |
29.22 |
31.97 |
|
9.50 |
26.94 |
29.17 |
|
19.50 |
16.25 |
19.31 |
|
24.50 |
12.66 |
16.44 |
|
29.50 |
9.52 |
13.49 |
|
41.50 |
5.42 |
10.06 |
|
69.50 |
1.55 |
3.83 |
|
116.50 |
|
2.05 |
2. Prepare a Hammett Plot for
the chlorination of phenol at high pH (i.e., phenate ions). Use all of the rate constants in Figure 13
from Deborde & von Gunten, 2008 [Water
Research 42:13-51]. To help you with
this you should number each carbon and treat the attack on different carbon
atoms separately even if they result in the same product. Use the Hammett substituent constants in the
attached table. Compare your results
with those obtained by Deborde & von Gunten in their Figure 15.
3. Use the Hammett Plot prepared
for #2, and predict the rate constant for the reaction of chlorine with the
phenate ion of 2-amino-4-nitro-5-methyl phenol.
Assume that the two unsubsituted carbons (C3 and C6) are the only sites
of attack. Estimate the relative rate of
attack on these two carbons.
Assigned: 1 Dec10
Due: 10 Dec 10
Hammett Substituent Constants
|
Substituent |
σp |
σm |
σo |
σp+ |
σ+m |
σ* |
R |
F |
|
-N(CH3)2 |
-0.83 |
-0.16 |
-0.36 |
-1.70 |
|
|
-0.98 |
0.15 |
|
-O- |
-0.81 |
-0.47 |
-1.10 |
|
|
|
|
|
|
-NH2 |
-0.66 |
-0.15 |
0.03 |
|
|
0.10 |
-0.74 |
0.08 |
|
-OH |
-0.35 |
0.08 |
0.04 |
|
|
0.25 |
-0.70 |
0.33 |
|
-OCH3 |
-0.26 |
0.08 |
0.00 |
-0.76 |
0.05 |
0.25 |
-0.56 |
0.29 |
|
-C(CH3)3 |
-0.20 |
-0.10 |
-0.52 |
-0.26 |
|
|
-0.18 |
-0.02 |
|
-CH3 |
-0.16 |
-0.07 |
-0.13 |
-0.31 |
-0.06 |
-0.05 |
-0.18 |
0.01 |
|
-CH(CH3)2 |
-0.15 |
-0.04 |
-0.23 |
-0.28 |
|
|
-0.19 |
0.04 |
|
-CH2C6H5 |
-0.09 |
-0.08 |
|
-0.28 |
|
|
-0.05 |
-0.04 |
|
-CH=CHC6H5 |
-0.07 |
0.03 |
|
-1.00 |
|
|
-0.17 |
0.10 |
|
-CH=CH2 |
-0.04 |
0.06 |
|
-0.16 |
|
|
-0.17 |
0.13 |
|
-OC6H5 |
-0.03 |
0.25 |
|
-0.50 |
|
|
-0.40 |
0.37 |
|
-C6H5 |
-0.01 |
0.06 |
0.00 |
-0.18 |
0.11 |
0.10 |
-0.13 |
0.12 |
|
-H |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
-NHCOCH3 |
0.00 |
0.21 |
|
-0.60 |
|
|
-0.31 |
0.31 |
|
-CH2OH |
0.01 |
0.01 |
0.04 |
|
|
|
|
|
|
-F |
0.08 |
0.35 |
0.54 |
-0.07 |
0.35 |
0.52 |
-0.39 |
0.45 |
|
-Cl |
0.23 |
0.37 |
0.68 |
0.11 |
0.40 |
0.47 |
-0.19 |
0.42 |
|
-Br |
0.23 |
0.39 |
0.70 |
0.15 |
0.41 |
0.45 |
-0.22 |
0.45 |
|
-I |
0.28 |
0.35 |
0.63 |
0.14 |
0.36 |
0.39 |
-0.24 |
0.42 |
|
-CONH2 |
0.36 |
0.28 |
0.72 |
|
|
|
0.10 |
0.26 |
|
-CHO |
0.42 |
0.35 |
0.75 |
0.73 |
|
|
0.09 |
0.33 |
|
-COC6H5 |
0.43 |
0.34 |
|
0.51 |
|
|
0.12 |
0.31 |
|
-COOCH3 |
0.45 |
0.36 |
|
0.49 |
|
|
0.11 |
0.34 |
|
-COCH3 |
0.50 |
0.38 |
|
|
|
|
0.17 |
0.33 |
|
-CN |
0.68 |
0.62 |
1.32 |
0.66 |
0.56 |
0.58 |
0.15 |
0.51 |
|
-CH3SO2 |
0.71 |
0.65 |
|
|
|
0.59 |
|
|
|
-NO2 |
0.79 |
0.71 |
1.40 |
0.79 |
0.67 |
0.63 |
0.13 |
0.65 |
Note
that σo values are estimated for
phenols; source: Appendix A5 in Perrin, Dempsey & Serjeant, 1981, pKa
Prediction for Organic Acids and Bases, Chapman & Hall.