CHAPTER VII
ANTHROPOGENIC COMPOUNDS
A. INTRODUCTION
So many compounds
that have been found to be harmful to humans or to some aspect of nature owe
their presence in the biosphere in large part to human activities. Sometimes its a case of relocating a chemical
substance from an environment where it cannot cause any harm to a place where
it will. In many cases, however,
completely new compounds are formed.
Man-made compounds that do not normally exist in nature are called xenobiotics. In other cases compounds will be formed
whereby further examination reveals the existance of natural processes that
result in their formation. When most of
the total mass of a harmful chemical released into some environment can be attributed
to human activities, it is said to be an anthropogenic pollutant.
B. NATURALLY-OCCURRING POLLUTANTS
There are some types of naturally-occurring substances
that are also considered anthropogenic pollutants. Although they were not created by humans, they were extracted and
re-distributed by them. Generally this
means that these compounds were placed in environments where they could easily
come into contact with living organisms.
Whereas previously they were isolated, with far less opportunity for
contact with environments that might be harmed by them. A few examples of such naturally-occurring
pollutants are petroleum products and heavy metals.
1. Petroleum Products
Petroleum or fossil
fuels are naturally-occurring complex mixtures of organic compounds, mostly
hydrocarbons. The compounds in crude
petroleum may range in molecular weight from 16 (methane) up to 20,000 and
above. Petroleum may also contain small
amounts of oxygen, sulfur and nitrogen as well as the aforementioned carbon and
hydrogen.
The precise
composition of this enormously complex mixture is somewhat site specific. Nevertheless, it is useful to classify the
constituent organics into three groups:
aliphatics
alicyclics
aromatics
The aromatic component is often dominated by the BTEX compounds:
Benzene, Toluene Ethylbenzene and Xylene.
There are many
methods for analysis of petroleum hydrocarbons. These differ in cost, method detection limit (MDL) and extent of
characterization possible (Table 7.1).
The non-specific or non-qualitative methods (the first 3 in table 7.1)
are primarily used as a screening tool and for mapping the size and movement of
petroleum plumes. They can only provide
a single-number assessment of the total hydrocarbon concentration. Detailed site investigations require the use
of one or more gas chromatographic (GC) methods. These are capable of providing fingerprints of the hydrocarbon
mixture or even compound-specific concentrations.
Table 7.1
Methods for Analysis of
Liquid-Phase Petroleum Hydrocarbons
(from DEP, 1994)
|
Method |
Approx. MDL (µg/L) |
Approx. Cost ($) |
also Qualitative |
Identification & Confirmation |
|
Gravimetric |
10,000 |
50 |
No |
No |
|
Infra-red |
2000 |
65 |
No |
No |
|
UV Fluroescence |
10 |
50 |
No |
No |
|
GC/FID Screen |
10,000 |
125 |
Yes |
No |
|
GC with PID/FID |
1 |
175 |
Yes |
No |
|
GC/FID with clean-up |
100 |
250 |
Yes |
No |
|
GC/MS |
10 |
700 |
Yes |
Yes |
Crude petroleum is
refined to give a wide range of useful fractions. Some of these are discussed below.
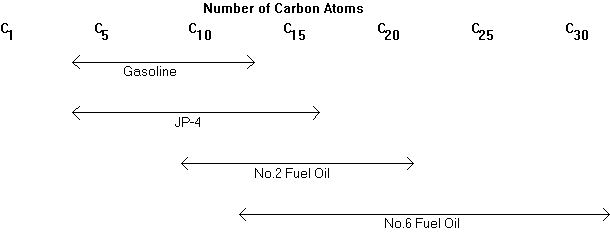
Figure 7.1
Typical Size Ranges for
Petroleum Products
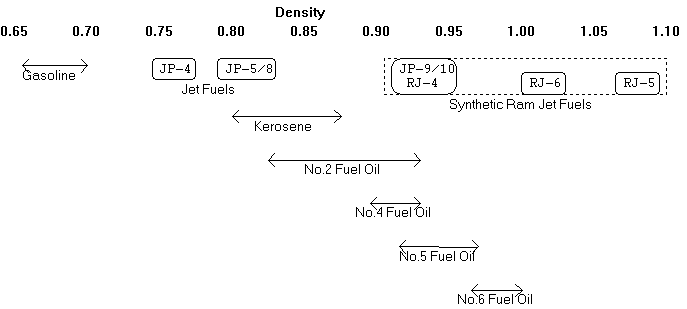 Figure 7.2
Figure 7.2
Typical Densities for
Petroleum Products
a. Gasoline
Gasoline
is a blend of light hydrocarbon fractions from petroleum. There are the familiar automotive gasolines
as well as aviation gasoline (Av Gas), used in piston-driven propeller
aircraft. Virgin gasoline is probably
about 50% alkanes, 40% cycloalkanes and 10% aromatics. However, this is blended with catalytically
cracked gasoline and thermally reformed gasolines at the refinery. The blending process substantially changes
the makeup, elevating the alkene content up to as much as 30%. Blending is done with the very practical
objective of improving performance in combustion engines. Gasoline may contain a number of additives
aimed at improving certain aspects of performance. These include anti-knock agents (or octane enhancers: e.g.,
methyl t-butyl ether [MTBE] and some alcohols), antioxidants
(N,N'-dialkylphenylenediamines, and some phenols), metal deactivators
(N,N'-disalicylidene-1,2-ethanediamine and related compounds), lead scavengers,
anti-rust agents (carboxylic acids, phosphoric acids), anti-icing agents
(isopropanol), upper cylinder lubricants, detergents and dyes. In particular, the use of MTBE is
significant. Unleaded gasoline accounts
for nearly all of the MTBE use in the U.S.
It contrasts with many of the other gasoline constituents in that it is
highly water soluble, while still quite volatile and resistant to biodegradation. As a result, MTBE has a high potential for
exposure to humans by a water route, and especially following volatilization
from water.
Since,
gasoline is not blended to any specific chemical composition, its precise
makeup is hard to characterize. Of all
the major petroleum products, gasoline probably has the highest alkane content,
a low alicyclic content, and a low to medium aromatic content. It differs from jet fuel in that the latter
is higher in alicyclics and lower in alkanes.
The fuel oils have higher aromatic contents, average alicyclic contents
and very low levels of alkanes. Refer
to chapter III for a listing of the major constituents in two commercial
automotive gasolines. A general
accounting by compound type is in Table 7.2.
This breakdown is highly variable.
Table 7.2
Typical Components of
Unleaded Automotive Gasoline
(from Domask, 1983)
|
Type |
Carbon # |
No. of Isomers |
% by |
Major Contributors |
||
|
|
|
Possible |
Analyzed |
Volume |
Number |
% of total |
|
n-alkanes |
C3-C10 |
8 |
8 |
11.4 |
3 |
10.2 |
|
isoalkanes |
C4-C13 |
>600 |
51 |
46.5 |
17 |
38.0 |
|
cycloalkanes |
C5-C13 |
>600 |
49 |
4.7 |
5 |
1.9 |
|
mono-alkenes |
C2-C12 |
>600 |
29 |
9.0 |
8 |
3.3 |
|
aromatics |
C6-C13 |
>200 |
14 |
28.4 |
9 |
20.8 |
|
TOTAL |
|
>2000 |
151 |
100.0 |
42 |
74.2 |
b. Naphtha or Light Petroleum Distillates
These are fractions
that include mineral spirits, gasoline blending stocks and a wide range of
petroleum solvents.
c. Kerosene
Kerosene is heavier
than gasoline, but still considered a light distillate. Since it is used in vaporizing burners, it
is designed to have a high volatility.
Kerosene is a straight-run distillate, prepared without blending. Petroleum products that are largely kerosene
include Fuel Oil No. 1 and Diesel Fuel No. 1.
The diesel fuel contains some special additives. Kerosene's typical composition is: 35% alkanes,
60% cycloalkanes, and 15% aromatics.
d. Fuel Oils
These are broad
mixtures having properties defined by ASTM standars. Number 1 fuel oil is actually in the kerosene category. The category of fuel oil represented by
number 3 has now been included in number 2.
Numbers 4, 5 and 6 are collectively called the heavy fuel oils (HFO), or
residual fuel oils.
Fuel Oil Number 2 is
also known as heating oil or diesel oil or diesel fuel no.2. It is designed for use in atomizing burners,
such as those found in homes. It is
easier to handle but more costly than the residual fuel oils. The ASTM has defined three grades; 1-D, 2-D,
and 4-D; of increasing weight. The
average composition of these oils is: 30% alkanes, 45% cycloalkanes, and 25%
aromatics.
Fuel Oil Number 4 is
used for higher viscosity atomizing burners.
However, it generally does not require pre-heating.
Fuel Oil Number 5 is
so viscous that it requires heating prior to pumping.
Fuel Oil Number 6 is
commonly called Bunker C Fuel Oil. It
is obtained by blending residues from cracking or distillation with certain
distillates. Like #5, this mixture
requires heating prior to use. Use of
this oil is only practical in large industrial settings. Fuel Oil Number 6 is typically composed of
about: 34% aromatics, and 20% alkanes.
It also contains substantial amounts of 3 to 7-ring polynuclear aromatic
hydrocarbons (PAHs); from 6 to 20% or more.
e. Turbo and Missile Fuels
These include a range
of turbo jet fuels and ram jet fuels designed for use in gas turbine-powered
aircraft and missles and ramjet-powered missles. These have different properties than aviation gasoline which
fuels piston-engine aircraft. The
majority of the jet fuels are composed of distillates which are quite similar
to Kerosene and Fuel Oil Number 1 (JP-4, JP-5 and JP-8). JP-4 is blended with the more volatile
gasoline fractions. This fuel contains
as much as 80% alkanes, with 20% aromatics making up the rest. Both JP-5 and JP-8 are composed of
kerosene-based distillates, and resemble Commercial Jet A-1 fuel. Like gasoline, jet fuels can contain a
number of additives.
The higher density
ram jet fuels are all synthetic, and are only included here because they are
used like distillate jet fuels. Three
of these are made from pure compounds: exo-tetrahydrodi(cyclopentadiene) is
JP-10; tetrahydrodi(methylcyclopentadiene) is RJ-4, and
endo,endo-dihydro(norbornadiene) is RJ-5.
JP-9 is a blend of methylcyclohexane, JP-10 and RJ-5. RJ-6 is a blend of RJ-5 and JP-10.
f. Lubricating Oils and Crankcase Oils
These are fractions
that have been reduced in their aromatic content. Aromatics result in poorer viscosity characteristics and are more
susceptible to unwanted oxidation. Their
general composition is: 45-76% alkanes, 13-45% cycloalkanes, adn 10-30%
aromatics. Used crankcase oils often
have elevated levels of polynuclear aromatic hydrocarbons (PAHs) produced by
combustion processes (see part D, below).
They may also contain heavy metals from engine wear.
2. Heavy Metals
Many contaminated
groundwaters contain elevated concentrations of heavy metals. Among the more frequently reported metals
are lead, chromium, arsenic and zinc. Drinking water standards
currently exist in the US for aluminum, antimony, barium, beryllium, cadmium,
chromium, copper, lead, mercury, nickel, selenium, and thallium. Metals in drinking water may be grouped into
three classes: (1) those that are present in the raw water and incompletely
removed through treatment, (2) those that are purposely added over the course
of treatment, and (3) the corrosion byproducts. The second group primarily includes aluminum. Members of this last group include lead,
copper, and to a lesser extent, cadmium and chromium. The transition metals are generally less volatile than other
water contaminants, although they may be transported by aerosol formation and
even dry deposition.
Although
aluminum is one of the most abundant elements in the earth’s crust, it is
rarely present in water at high concentrations. This is due to its strong tendency to form insoluble
hydroxides. For this reason, addition
of aluminum in the form of alum for the treatment of water does not generally
lead to elevated levels of the metal in finished water. However, problems may occur when the pH of
the water drops to low (e.g., 5 or below).
Under these conditions, aluminum hydroxide precipitates will not form to
as great an extent, and aluminum solubility rises sharply.
Antimony
has toxic effects, which manifest themselves in the gastrointestinal tract, the
cardiovascular system, the skin and liver.
It exists in two oxidation states, (+III) and (+V). Antimony is normally present at very low
concentrations in water, and as a result, little is known about its
speciation. Of the 50 elements studied
by the US Geological Survey in soil and rock samples, antimony was the third
least abundant, with an average composition of 0.5 ppm (Shacklette and Boerngen
1984). Antimony may become methylated in aquatic
environments, which can substantially elevated its solubility (Andreae 1983).
Barium
readily forms insoluble sulfate and carbonate precipitates, which renders the
metal highly insoluble. For this reason
alone, barium is not considered a major threat to human health. Barium is, however, a common contaminant at
hazardous waste sites in the US.
Beryllium
is not widely found in the natural environment. The presence of high concentrations of this metal suggest
contamination from industrial wastewater, especially non-ferrous metal
manufacturers. The atmosphere may also
serve as an important Beryllium source for natural waters (EPA 1987). The US EPA has
determined that Beryllium is a probable human carcinogen.
Cadmium
can accumulate in the kidneys, eventually causing damage to that organ, and it
can also cause bones to become fragile and break. The US EPA has determined that cadmium is a probable human
carcinogen by inhalation. Water is
rarely a major route of exposure to cadmium; food and incidental ingestion of
dust, being much more important.
However, in some instances, significant amounts of cadmium have been
found to leach from plumbing fixtures.
Chromium
exists in water in either the +III or the +VI oxidation state. Although chromium is an essential nutrient
for the human diet, high atmospheric levels of Cr(+VI) have been associated
with lung cancer in workers. For this
reason, there is some concern over the hexavalent form. Chromium will adsorb to metal oxides and
form strong chelates in natural waters.
As a result, it is primarily found in water in a particulate form, with some
soluble chromium complexes. The mean US
chromium level for river water has been estimated at 10 mg/L(Eckel and Jacob 1988). Concentrations in
tap water have been found to be lower (1.8 mg/L average,Greathouse and Craun 1978), but corrosion of plumbing fixtures can drive these
up. Some contaminated groundwaters may
contain very high levels of chromium.
Copper
is one of those metals that is primarily present in tap water as a result of
corrosion of pipes and plumbing fixtures.
While copper corrosion is a nuisance, it is rarely a human health
concern. However, those afflicted by
Wilson’s disease (a copper metabolism disorder) may find levels of a few mg/L
intolerable.
Lead
can be a major concern in water systems subject to high levels of
corrosion. Lead can cause damage to the
brain and kidneys. In children, it can
be especially damaging to mental development.
Because lead readily precipitates as carbonates and hydroxides, it is
rarely present at high concentrations in natural waters. Nevertheless, elevated lead levels can be
found in tap water if the water is corrosive and if there is contact with lead
in the pipes. Possible sources of lead
include lead service connections, lead-tin solder and lead-containing brass
fixtures. As with all corrosion
byproducts, concentrations of lead are highest at the tap during the “first
flush” of water in the morning.
Mercury
can lead to permanent damage of the brain, kidneys, and the growing fetus. Mercury exists in three oxidation states (0,
+I, +II). Metallic-mercury readily
volatilizes and can be transported through the atmosphere. Under reducing conditions, certain
microorganisms, such as the sulfate-reducing bacteria, can methylate mercury,
which further aids its transport (Gilmour and Henry 1991). Mercury is
characterized by its ability to form strong complexes and concentrate through
the food chain.
Nickel
is another trace metal that is thought to be an essential human nutrient. It does not appear to be very toxic, but it
is probably carcinogenic when inhaled in the form of dust. Nickel is strongly bound to particle
surfaces in water, and therefore is rarely present at high dissolved
levels. It is not as readily concentrated
through the food chain as other metals, such as mercury, can be. Unpolluted surface and groundwaters have
nickel concentrations in the low ppb range (Shiller and Boyle 1987;Page 1981). In general,
drinking waters have similar levels, although in at least one study (Ohanian 1986) evidence was found for contributions from corrosion.
Thallium
can adversely affect the human nervous system, lung, heart, liver and
kidney. Amounts as small as 1 gram can
be fatal. Thallium is most commonly
found in the monovalent form, and it tends to be bound to soil particles. It is normally present in very low
concentrations in water; below 1 ppb in tap water (EPA 1988). When higher
concentrations are found, it is usually due to contamination from mining
operations or metal manufacturing wastewaters.
Radionuclides
The
major radionuclides of concern in drinking waters are radium 226 and 228,
uranium, and radon 222. Because it is a
gas, radon poses a special concern. It
is found in many groundwaters, depending on the local geology, and its
concentrations can be highly variable.
Based on data from the National Inorganic and Radionuclides Survey, 17
million Americans are expected to have radon levels in their water in excess of
the MCL (300 pCi/L), and 2.7 million are thought to have water above 1,000
pCi/L.
Inorganic Non-metals
Nitrogen
species can present toxicity to humans under certain circumstances. One of the best known examples is the
condition known as methemoglobinemia.
This occurs when infants ingest water with high levels of nitrate or
nitrite.
Selenium
is toxic at high doses, yet it is also an essential nutrient. For this reason, its regulation is quite
complex. Symptoms of excessive selenium
uptake include brittle hair and deformed nails. From a chemical standpoint, selenium behaves much like
sulfur. It has four oxidation states
(-II, 0, +IV, +VI), and the two most oxidized states tend to form the more
soluble compounds. Both of these forms
(selenites and selenates) are common in aerated waters. Selenium is relatively bioactive, and
microorganisms, such as cyanobacteria are known to be able to methylate the
metal (Bender et al. 1991).
C. SYNTHETIC ORGANIC CHEMICALS
1. Industrial Solvents
a. Chlorinated Solvents
These compounds
include trichloroethylene, tetrachloroethylene, 1,1,1-trichloroethane,
dichloromethane (methylene chloride), chloroform, carbon tetrachloride and many
more (Figure 7.3). They are all
produced in large quantities for industrial use. Furthermore, they are relatively persistent and mobile,
especially in the subsurface. All of
these 1 and 2-carbon haloaliphatics are readily volatilized from water. For this reason, they are considered members
of the group, volatile organic compounds or VOCs.
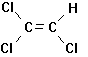
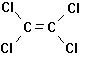
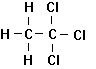
Trichloroethylene Tetrachloroethylene 1,1,1-Trichlorethane
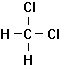
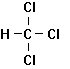
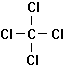
Dichloromethane Chloroform Carbon Tetrachloride
Figure 7.3
Some Common
Industrial Chlorinated Solvents
Dichloromethane is
used in paint strippers, and as a decaffeinating solvent. Chloroform was used as an anesthetic around
the turn of the century. Like its
cousin, carbon tetrachloride, it is a good general solvent. Production of the common solvent,
1,1,1-trichloroethane is being phased out due to its detrimental effect on the
stratospheric ozone layer. This
chemical is a popular degreaser and can be found in household glues, spot
cleaners and aerosol sprays.
Tetrachloroethylene (also known as perchloroethylene) has replaced
carbon tetrachloride as the solvent of choice among dry cleaners.
2. Propellants and Refrigerants
These encompass
primarily the chloro-fluoromethanes: trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and
chlorodifluoromethane (CHClF2). As a group they are called
chlorofluorocarbons (CFCs). Although,
small amounts of the CFCs may be produced by volcanoes, they are primarily
man-made. They are being phased out of
use in favor of compounds that are less harmful to the ozone layer.
3. Pesticides
Pesticides may be
used to control a wide range of plants and animals deemed undesirable. Most are either chlorinated compounds (e.g.,
DDT, methoxychlor, mirex, lindane, endrin, 2,4-D, 2,4,5-T), organo-phosphorus
compounds (e.g., parathion, malathion), triazines (e.g., atrazine) or
carbamates. These are all man-made
chemicals. Some of the more current
occurrence data may be found in Thurman 1992 and Stamer 1996.
One of the most
celebrated of these compounds is DDT.
Although it was first synthesized as early as 1873, it wasn't until 1939
when Paul Muller of J.R. Geigy studied the compound that its utility as a
pesticide was fully recognized. DDT
found widespread use during World War II, helping to control the insects that
spread Typus, Malaria, Typhoid and Dysertery.
Later, in 1948 Muller was awarded a Nobel Prize for his work with DDT.
In general,
chlorinated organic compounds are associated with man-made pollutants. However, there are over 2000 known
naturally-produced halogenated organics (Gribble, 1994). Many marine and terrestrial organisms
produce halo-organics that serve as pesticides or related compounds which can
give the organism a feeding advantage.
4. Phthalates
Phthalates are esters
of phthalic acid. They are produced in
very large quantities, and used primarily as plasticizers. For example, they are used to make polyvinyl
chloride (PVC) flexible.
5. Surfactants
Surfactants are of
three major types: anionic, nonionic and cationic. The anionic surfactants comprise the soaps (sodium salts of
simple, aliphatic fatty acids), linear alkylbenzene sulfonates, secondary alkyl
sulfonates, and fatty alcohol sulfates
The nonionic surfactants are mostly polyethylene glycol ethers of a
phenol or an aliphatic alcohol. The
cationic surfactants are quaternary ammonium chloride derivatives.
6. Miscellaneous Synthetics
a. PCBs
The polychlorinated
biphenyls (PCBs) are aromatic hydrocarbons that have been used as capactor
dielectrics, transformer coolants, hydraulic fluids and heat transfer
fluids. These compounds all share the
biphenyl structure, with varying numbers of chlorine atoms (1-10) substituted
for hydrogens. For example, there would
be 12 unique arrangements for 2 chlorine atoms on the biphenyl structure. These would all be isomers of each
other. However, the total number of
possible structures of varying chlorine content is 209. Related compounds of this type are called congeners.
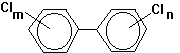
Most PCBs were
produced by one manufacturer (Monsanto Corp.) under the trade name,
Arochlor®. Actually are were mixtures
of PCBs which vary in the average chlorine content (see Table 7.3). The first two numbers in the numerical code
indicate the number of carbon atoms (12), and the last two indicate the average
percent chlorine (by weight). These
compounds were sold in large quantities in the US from 1930 to 1977.
Table 7.3
Average Composition of 6
Arochlors by Weight
(from Erickson, 1986)
|
Number of |
Arochlor |
|||||
|
Chlorines |
1221 |
1232 |
1242 |
1248 |
1254 |
1260 |
|
0 |
10 |
|
|
|
|
|
|
1 |
50 |
26 |
1 |
|
|
|
|
2 |
35 |
29 |
13 |
1 |
|
|
|
3 |
4 |
24 |
45 |
2 |
1 |
|
|
4 |
1 |
15 |
31 |
49 |
15 |
|
|
5 |
|
|
10 |
27 |
53 |
12 |
|
6 |
|
|
|
2 |
26 |
42 |
|
7 |
|
|
|
|
4 |
38 |
|
8 |
|
|
|
|
|
7 |
|
9 |
|
|
|
|
|
1 |
D. UNCONTROLLED REACTION BYPRODUCTS
1. Combustion Byproducts
a. Polycyclic Aromatic Hydrocarbons or Polynuclear Aromatic
Hydrocarbons (PAHs)
PAHs are common
byproducts of the combustion of fossil fuels, wood, cigarettes, and
charcoal-broiled foods. While they are
present in crude oils, and can be produced by natural combustion processes
(e.g., forest fires, volcanoes), it is the anthropogenic sources that are of
greatest concern. Several of these
compounds (e.g., anthracene, acenaphthene, fluorene, and phenanthrene) are
commercially prepared for use primarily in the manufacture of dyes.
Most of the PAHs in
natural surface waters are thought to have come from atmospheric
deposition. These compounds are
relatively insoluble. Accordingly, they
tend to readily adsorb to particles and sediments, or they can volatilize. Due to their insoluble, hydrophilic nature,
they accumulate in aquatic organisms, and readily move up the food chain.
Quite a few of the
PAHs studied are now classified by the US EPA as probably human carcinogens. This groups includes benzo(a)pyrene,
benzo(a)anthracene, benzo(b)fluoranthene and many others.
b. Dioxins
This group of
compounds is more correctly called the chlorodibenzo-p-dioxins. The most
important is probably 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD).
There are 22 isomers of TCDD.
The chlorinated dibenzodioxins and dibenzofurans may also be produced in
very small quantities by some animals (Gribble, 1994).
2. General Chlorination Byproducts
Active
chlorine is used as a disinfectant, a bleaching agent, a halogenating agent and
a general oxidant for a wide range of purposes. Most common to our everyday experience is chlorine's use in the
production of drinking water, and in swimming pools. However, chlorine is also used to bleach wood pulp in the
manufacture of paper, to disinfect municipal wastewaters, and in the synthesis
of many industrial chemicals. Since
chlorine is so reactive, it will attack many types of compound in water, not
just the target substance (e.g., pathogenic organisms, colored wood
fibers). Some of the organic byproducts
that are formed will contain organically-bound chlorine. Concern over the general toxicity of
organo-chlorine compunds has opened a national debate. Many are questioning the need for chlorine,
and proposing a national ban on chlorine's use. A well-studied example is that of the chlorination byproducts
produced during drinking water treatment.
These will be discussed in detail below.
Chlorine
reacts with naturally-occurring organic matter during drinking water treatment
to form a diverse group of organic byproducts; some hazardous, and some
not. The hazardous compounds that have
concerned the US EPA and state regulatory agencies are the halo-organic
mutagens or suspected carcinogens (Bull et al., 1982; Kringstad et al.,
1983). Researchers at the US EPA Health
Effects Laboratory in Cincinnati have been actively pursuing the identity of
these compounds. Their approach has
included the use of the Ames mutagenicity test in conjunction with GC/MS
analysis to determine the specific compounds responsible for the mutagenic
activity in chlorinated natural waters.
Despite extensive studies of this type, only 7-8% of the overall
mutagenicity in chlorinated humic acid solutions could be accounted for by 1985
(Meier et al., 1985). The discovery, in
1986, of low concentrations of an extremely potent mutagen,
3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (more commonly referred to
as MX, see equation 1), in chlorinated humic acid solutions and drinking waters
has significantly advanced this effort (Coleman et al., 1986). In a survey of 6 chlorinated drinking
waters, MX alone was found to account for 22%-44% of the overall mutagenicity
(Holmbom et al., 1987). A subsequent
study of 26 chlorinated Finnish waters found MX to account for 15-57% of the
overall mutagenicity (Kronberg & Vartianinen, 1988). The likely effects of MX on humans still
remains to be determined (Daniel et al., 1994). The next most important mutagen may be 2,3,3-trichloroprepenal. This compound may account for 4% of the
overall mutagenic activity from chlorinated humic acid solutions (Meier et al.,
1985), although it has not yet been isolated from drinking waters. Despite recent advances in this area, more
than 50% of the mutagenicity of chlorinated drinking waters remains unaccounted
for.
Others
researchers have identified and quantified numerous halo-organics in an effort
to account for all of the Total Organic Halide (TOX) produced by drinking water
chlorination. Table 6 summarizes some
of these findings. In this endeavor,
roughly 50% of the TOX has been identified (Christman et al., 1983).
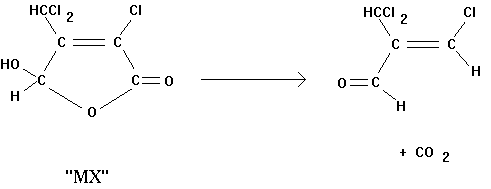 (1)
(1)
Table 6
Major Halo-organic Byproducts of Chlorination
|
Compound(s) |
% of TOX |
Early References |
|
Trihalomethanes |
15 - 25 |
Sander et al., 1977 |
|
Trichloroacetic Acid |
5 - 20 |
Reckhow & Singer, 1984, Norwood et al., 1986 |
|
Dichloroacetic Acid |
5 |
Christman et al., 1983; Reckhow & Singer, 1984 |
|
Other Aliphatic Chloroacids |
|
Norwood et al., 1980; De Leer et al., 1985 |
|
1,1,1-Trichloropropanone |
0.8 |
Coleman et al., 1984; Reckhow & Singer, 1984 |
|
1,1-Dichloroacetone |
0.2 |
Coleman et al., 1984 |
|
Other Aliphatic Haloketones |
|
Coleman et al., 1984; Sato et al., 1985 |
|
Aliphatic Chloroaldehydes |
|
Kopfler et al., 1985 |
|
Dihaloacetonitriles |
0.5 |
Reckhow & Singer, 1984; Oliver, 1983 |
|
Other halonitriles |
|
Coleman et al., 1984 |
|
Halophenols |
|
Coleman et al., 1984; Kringstad et al., 1985 |
|
Chloroaromatic Acids |
|
De Leer et al., 1985; Seeger et al., 1985 |
|
Halothiophenes |
|
Coleman et al., 1984 |
|
Chlorinated PAHs |
|
Shiraishi et al., 1985 |
The
reason that efforts to identify all of the chlorination byproducts has not been
100% successful is two-fold. First,
there may be many chlorination byproducts that are not readily amenable to gas
chromatography (e.g., complex, high molecular weight compounds). Second, many of the byproducts may not be
sufficiently stable to survive the various extraction, separation and
derivatization procedures used, as well as the high injector temperatures often
employed. Indeed, Kopfler and
co-workers (1985) found that 50% of the mutagenic activity of chlorinated humic
acids decomposed upon heating (in aqueous solution) to 220oC for one
minute. Others have noted a complete
loss at 250oC (Kool et al., 1985). Kopfler and co-workers also found that less than 10% of the
original mutagenic activity was recoverable by trapping all of material in the
GC column effluent. One important heat-labile
compound is the potent mutagen, MX.
This important chlorination byproduct undergoes complete decarboxylation
to form 3-chloro-2-(dichloromethyl) propenal, which is probably far less
mutagenic that the parent compound (see equation 1, below).
The
trihalomethanes are the best known of the chlorination byproducts (see Figure
8). They are also the most easily
measured. Chloroform has been shown to
cause cancer in laboratory animals and the other trihalomethanes (THMs) have
been found to be mutagenic in the Ames Salmonella assay. The chloroacetic acids are also widespread,
with the di and tri compounds being found in all chlorinated waters. Although dichloroacetic acid (DCAA) and
trichloroactic acid (TCAA) are not apparently mutagenic, some preliminary
evidence indicates that they may be carcinogenic to laboratory animals (NRC,
1987).
Trihalomethanes (THMs)

Haloacids (HAAs)
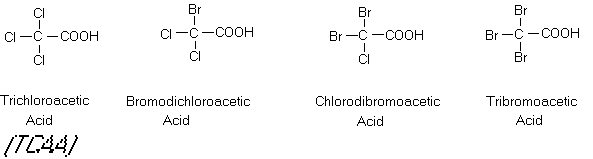
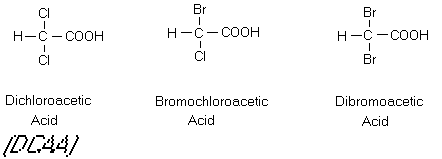
Figure 8
The Trihalomethanes and Chloroacetic Acids
The
haloacetonitriles (Figure 9) are thought to be products of the chlorination of
amino acids and proteinaceous material (Trehy & Beiber, 1981). In addition, de Leer and co-workers (1985)
have shown that isolated humic materials react with chlorine to give cyano
acids which continue to react to form haloacetonitriles. Dichloracetonitrile and
bromochloroacetonitrile have been determined to be direct acting mutagens. Trichloroacetonitrile,
bromochloroacetonitrile and dibromoacetonitrile all showed varying
carcinogenicities in tests with laboratory animals. Another N-containing chloro-organic, chloropicrin (see Figure
11), was found to be an indirect acting mutagen and proved negative in several
carcinogenicity tests.
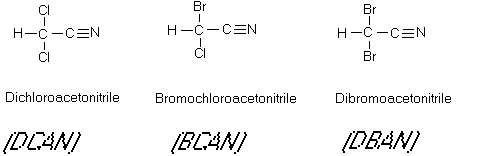
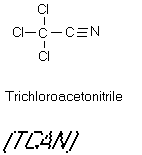
Figure 9
The Haloacetonitriles
Among the chlorination byproducts of concern are many
halogenated aldehydes and ketones (see Figure 10). Chloral is a mutagen although its carcinogenicity is
uncertain. 1,1,3-Trichloropropanone,
1,1,3,3-tetrachloropropanone, pentachloropropanone and hexachloropropanone were
all found to be direct acting mutagens.
They have not yet been tested for carcinogenicity.
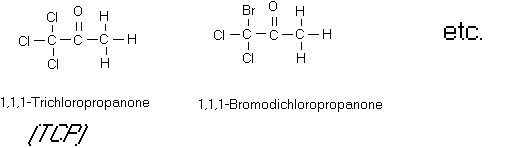
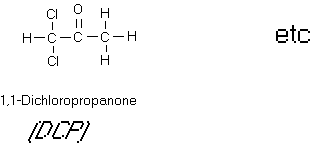
Figure 10
Some Chloroketones
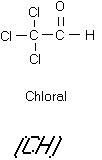
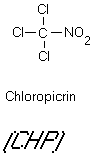
Figure 11
Chloral and Chlorpicrin
An extensive list of C3-C10 acids have
been isolated as chlorination byproducts of aquatic humic materials. Figure 12 shows some of the more common low
molecular weight members of this group.
Dichlorosuccinic acid may be the most prevalent halogenated byproduct
containing more than three carbon atoms.
CH3 Cl2COOH CCl2=CHCOOH CCl2=CClCOOH
2,2-Dichloropropanoic 3,3-Dichloropropenoic 3,3-Dichloropropenoic
Acid Acid Acid
HOOCCH2CHClCOOH HOOCCH2CCl2COOH
Chlorosuccinic
Acid Dichlorosuccinic
Acid
HOOCCH=CClCOOH HOOCCCl=CClCOOH HOOCCCl=CClCOOH
Chloromaleic
Acid Dichloromaleic
Acid Dichlorofumaric
Acid
Figure 12
Miscellaneous Chloroaliphatic Acids
A limited
amount of work has been done on chlorination byproducts not containing
carbon-halogen bonds. Chlorine is known
to react with a wide range of organic amines to form N-chloro-organic compounds
(i.e., organic chloramines; Morris, 1967).
The significance of these byproducts to human health is unknown. Because the TOX only represents a small
fraction of the total chlorine demand, most of the organic chlorination
byproducts probably do not contain chlorine.
A number of these non-halogenated byproducts have been identified
(Christman et al., 1983). Most are
aliphatic mono- and di-acids, and benzenepolycarboxylic acids. Because these compounds are probably not of
health concern, they have not been extensively studied. It should be noted that many of these
compouds are quite biodegradable, and therefore contribute to a water's
Assimilable Organic Carbon (AOC).
In
addition to the toxic chlorination byproducts there are others that may be
classified as aesthetically unpleasing; compounds that impart a taste &
odor to finished water. In an indirect
way, tastes and odors are also a health concern. Consumers may reject a disagreeable, yet safe, treated water for
a better tasting and unsafe substitute.
Chlorophenols have long been known as offensive byproducts of
chlorination (Burttschell et al., 1959).
Similarly, the chlorination of naturally-occurring phenolic type
precursors has been blamed for chronic taste and odor problems (Walker et al.,
1986). While chlorination byproducts
have been indicated as the source for many taste and odor problems, the
identities of the troublesome compounds are generally not well known (Jestin et
al., 1986). Nevertheless, it is clear
that chlorine taste is most closely related to chlorine demand (i.e., to
formation of organic byproducts), rather than to residual chlorine
concentrations (Bablon et al., 1986).
2. Chloramine Byproducts
Inorganic
chloramines are formed by the reaction of free chlorine with ammonia. The reaction is stepwise, giving
monochloramine (equation 2) followed by dichloramine (equation 3). The dichloramine is quite unstable, forming
nitrogen gas (equation 4) and some nitrate.
This decomposition is responsible for the classic breakpoint
chlorination phenomenon.
NH3
+ HOCl --------> NH2Cl + H2O (2)
NH2Cl
+ HOCl --------> NHCl2 + H2O (3)
NHCl2
+ NHCl2 + H2O
--------> HOCl + 3H+ +
3Cl- + N2 (4)
Normally
when chloramines are prepared "in situ" by the addition of ammonia
followed by chlorine, reaction 2 occurs so fast as to preclude any direct
reaction of free chlorine with organic matter.
If, however, the reverse order is used (i.e., chlorine then ammonia)
some free chlorination byproducts may be formed before the hypochlorous acid is
consumed by added ammonia.
Monochloramine
is known to add chlorine to the nitrogen of amines and amino acids forming
organic chloramines (Crochet & Kovacic, 1973). This reaction also occurs with free chlorine. Model compound studies have shown that
monochloramine can add chlorine to activated aliphatic carbon-carbon double
bonds (Minisci & Galli, 1965). The
adjacent carbon may become substituted with an amine group or with oxygen. Other types of reactions involve the simple
addition of amine or chloramine.
Chlorine substitution on to activated aromatic rings has been
observed. For example, monochloramine
will slowly form chlorophenols from monochloramine (Burttshcell et al.,
1959). Unfortunately, many of these
studies were carried out at extremes of pH or monochloramine concentration, and
their relevance to drinking water treatment conditions is uncertain.
Monochloramine
is far less reactive with most organic compounds than free chlorine. Studies with aquatic fulvic acid have shown
24-hour oxidant demands of 0.24 moles/mole carbon for chlorine and 0.06
mole/mole carbon for monochloramine (Jensen et al., 1985). About 100 times as much monochloramine was
required to achieve the same degree of fulvic acid bleaching as free
chlorine. The bleaching of humic
substances is probably related to the destruction of carbon-carbon double bonds. Identifiable byproducts include dichloroacetic
acid, cyanogen chloride and very small amounts of chloroform and
trichloroacetic acid. While its not
clear that TCAA and the THMs are true byproducts of chloramines (i.e., they may
be formed due to the presence of a small free chlorine residual), it does seem
that DCAA and cyanogen chloride are true byproducts. Backlund and co-workers (1988) found that monochloramine also
formed MX from humic materials, although the amount measured was less than 25%
of that formed during chlorination.
Mutagenic activity was less than 50% of the level produced by
chlorine. Shank and Whittaker (1988)
have proposed that small amounts of the known carcinogen, hydrazine (H2N-NH2),
may be formed when drinking waters are chloraminated. However, they have not yet been able to detect this compound in
drinking water distribution systems.
Jensen
and co-workers (1985) found that at high monochloramine/carbon mole ratios
(~10) about 5% of the oxidant demand ended up as TOX. This is identical to the results for chlorine at an equally high
chlorine/carbon ratio. At lower ratios
more typical of drinking water treatment (i.e., 0.2) the percent TOX to oxidant
demand for chlorine rises to 10-20%. There
is no reason to believe that would not also be the case for monochloramine. In other words, to get the same degree of
oxidation with monochloramine as with chlorine, one would have to apply a much
higher dose, and this higher dose would produce about the same amount of TOX. Most of the chlorinated organic byproducts
remain tied up in high molecular weight compounds. This contrasts with free chlorine, as monochloramine is a much
weaker oxidant and it is much less likely to oxidatively fragment humic
molecules to small ones that can be analyzed by gas chromatography.
3. Chlorine Dioxide
Byproducts
Chlorine
dioxide undergoes a wide variety of oxidation reactions with organic matter to
form oxidized organics and chlorite (equation 5). Chlorite may also be formed, along with chlorate (ClO3),
by the disproportionation of chlorine dioxide.
All three of these oxidized chlorine species (chlorine dioxide, chlorite
and chlorate) are considered to be toxic, and their presence in finished water
should be minimized.
ClO2
+ Organics ---------> ClO2 + oxidized organics (5)
Equation 5 represents a one electron transfer, and by
definition the oxidized organics contain a free radical (unpaired
electron). Model compound studies have
shown chlorine dioxide to be quite reactive with tertiary amines and phenols,
moderately reactive with olefins and slightly less reactive with alcohols and
aldehydes (NRC, 1980). Chlorine dioxide
has a greater tendency to react with alcohols and aldehydes (to form acids)
than other water treatment oxidant/disinfectants. Chlorine dioxide will not oxidize bromide to hypobromite, nor
will it react with ammonia.
Interestingly, it has been proposed that chlorite can react with certain
types of organic compounds to give hypochlorite as a byproduct.
Chlorine
dioxide will also undergo chlorine substitution reactions. Model compound studies have shown the
formation of chlorinated aromatic compounds and chlorinated aliphatics. Trihalomethanes, however, have not been
detected as reaction products. As with
the other chlorine-containing oxidants, chlorine addition/substitution products
are favored at low oxidant to carbon ratios and oxidation reactions are favored
at high ratios.
Very
little information exists on the chlorine dioxide byproducts from reaction with
humic substances. Experiments with
concentrated extracts of aquatic fulvic acid showed low reactivity, and very
little material that could be analyzed by gas chromatography (much less than
10% of the starting TOC; Colclough, 1981).
The major halogenated product was dichloroacetic acid, no
trichloroacetic acid was detected.
Chloromalonic and chlorosuccinic acids were also identified. Most of the identified byproducts consisted
of aliphatic diacids, simple aromatic acids and furan acids not containing
chlorine. In an earlier study using
Ohio River water treatment with chlorine dioxide resulted in the formation of
low molecular weight aliphatic aldehydes (Stevens et al., 1978). As with the model compound studies, no
trihalomethanes were detected from either study using natural aquatic
organic matter. Also, much less TOX is
produced as compared to what is normally found for chlorination (Rav-Acha et
al., 1983).
4. Ozonation Byproducts
Although
many researchers have applied GC/MS techniques for the purpose of identifying
organic ozonation products of raw waters and extracted humic materials, the
identifiable yields are generally very small (i.e., <5% of the starting
TOC). This is the case despite the very
high reactivity of ozone for humic substances.
Among the products reported are simple aldehydes (Schalekamp, 1977;
Trussell et al., 1981; Glaze et al., 1990), a large variety of low molecular
weight (MW) aliphatic acids, some keto-acids, hydroxy-acids, and benzene
polycarboxylic acids (Lawrence, 1977; Lawrence et al., 1980; Benga, 1980;
Paramisigamani et al., 1983; Anderson, 1984; Killops et al., 1985; Glaze,
1986). Among this group of identified
ozonation byproducts, perhaps, only the aldehydes are suspected as being human
health hazards (Glaze, 1986). In
particular, formaldehyde seems to be a ubiquitous product of drinking water
ozonation. In some waters, however,
this formaldehyde is diminished by reaction with other constituents (Glaze et
al., 1988). Since, the vast majority of
the ozonation byproducts are not gas chromatographable, toxicological assessments
of ozonation cannot be based solely on the identified compounds (Killops et
al., 1985).
The
tendency of ozone to form organic free radicals which can undergo subsequent
condensation reactions (i.e., higher MW) may also help to explain the lack of gas
chromatographable products. One group
of transient byproducts, the peroxides & hydroperoxides, cannot easily be
identified due to their high degree of reactivity. Studies employing general tests for oxidizing materials have
suggested that these compounds are present.
Research is currently underway using LC/MS to isolate and identify
organic peroxides.
LITERATURE
CITED
Babcock, D.B. and P.C.
Singer (1979) J. Am. Wat. Wrks. Assn., 71(3)149.
Bablon, G., C. Ventresque, F. Damez & M-C Hascoet (1986) AWWA
Annual Conference Proceedings, AWWA, Denver, CO, p1705.
Backlund, P., L. Kronberg, and L. Tikkanen (1988) "Formation of Ames
Mutagenicity of the Strong Bacterial Mutagen
3-Chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone and other Halogenated Compounds
during Disinfection of Humic Water," Chemosphere, 17:1327-1336.
Bull, R.J. & L.J. McCabe (1985) Water Chlorination: Chemistry,
Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis
Publ., Inc., Chelsea, MI, p111.
Bull, R.J., M. Robinson, J.R. Meier & J. Sorber (1982) Environ.
Health Pers., 46:215.
Burttschell, R.H., A.A. Rosen, F.M. Middleton & M.B. Etinger (1959) J.
Am. Wat. Wrks. Assn., 51(2)205.
de Leer, E.W.B., J.S.S. Damste, C. Erkelens & L. de Galan (1985) Envir.
Sci. & Technol., 19(6)512.
Carter, C.W. and I.H. Suffet (1982) "Binding of DDT to
Dissolved Humic Materials", Environ. Sci.& Technol.,
16:735-740.
Carter, C.W. and I.H. Suffet (1985) "Quantitative
Measurements of Pollutant Binding to Dissolved Humic Materials with Bulk
Properties of Humic Materials", Org. Geochem., 8(1)145-146.
Chiou, C.T., P.E. Porter and D.W. Schmedding (1983)
"Partition Equilibria of Nonionic Organic Compounds Between Soil Organic
Matter and Water", Environ. Sci & Technol., 17(4)227-231.
Christman, R.F., D.L. Norwood, D.S. Millington, J.D. Johnson & A.A.
Stevens (1983) Envir. Sci. & Technol., 17(10)625.
Colclough, C.A. (1981) "Organic Reaction Products of Chlorine
Dioxide and Natural Aquatic Fulvic Acids", MS Thesis, Univ. of North
Carolina, Chapel Hill, NC.
Coleman, W.E., J.W. Munch, W.H. Kaylor, R.P. Streicher, H.P. Ringhand
& J.R. Meier (1984) Environ. Sci. & Technol., 18(9)674.
Coleman, W.E., J.W. Munch, R.P. Streicher & P.A. Hodakievic (1986) 192nd
ACS Mtg, Division of Environmental Chem. Preprints, ACS, Washington, p332.
Condie, L.W. & J.P. Bercz (1985) Water Chlorination: Chemistry,
Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis
Publ., Inc., Chelsea, MI, p345.
Crochet, R.A. and P.
Kovacic (1973) J. Chem. Soc. Commun., 19:716.
Croue, J.-P. (1987) "Contribution a l'Etude de l'Oxydation par le
Chlore et l'Ozone d'Acides Fulviques Naturels Extraits d'Eaux de Surface",
Doctoral Thesis, University of Poitiers, France.
Daniel, F.B. et al. (1994) J. Am Wat. Wrks. Assn.
86(3)103-111.
De Haan, H., T. De Boer, H.A. Kramer and J. Voerman (1982) Water
Res., 16;1047-1050.
Edzwald, J.K., W.C. Becker and K.L. Wattier (1985)
"Surrogate Prarmeters for Monitoring Organic Matter and THM
Precursors", J. Am. Water Works Assn., 77(4)122-132.
Fielding, M. and H. Horth (1986) "Formation of Mutagens and
Chemicals During Water Treatment Chlorination," Water Supply,
4:103-126.
Gauthier, T.D., W.R. Seitz and C.L. Grant (1987) Environ.
Sci. & Technol., 21(3)243-252.
Glaze, W.H., L. Bauersachs and M. Gold (1988) "Oxidation in Water
Treatment: A Survey of Current Knowledge and Research Needs,"
Comprehensive Assessment report to AWWA Research Foundation, Denver, CO.
Gribble, G.W. (1994) "The Natural Production of Chlorinated Compounds,"
Environ. Sci. & Technol., 28:7:310A-319A.
Hall, E.S. and R.F.
Packham (1965) J. Am. Wat. Wrks. Assn., 57(9)1149-1166.
Handa, N. (1966) "Examination on the Applicability of
the Phenol Sulfuric Acid Method on the Determination of Dissolved Carbohydrate
in Sea Water, J. of the Oceanographic Soc. of Japan, 22:81-86.
Holmbom, B., L. Kronberg, P. Backlund, V.-A. Langvik, J.
Hemming, M. Reunanen, A. Smeds & L. Tikkanen (1987) presented at the Sixth
Conference on Water Chlorination, Oak Ridge, TN.
Holmbom, B., R.H. Voss, R.D. Mortimer & A. Wong (1984) Environ.
Sci. & Technol., 18(5)333.
Horth, H., M. Fielding, H.A. James, M.J. Thomas, T. Gibson, and P. Wilcox
(1988) "The Production of Organic Chemicals and Mutagens During
Chlorination of Amino Acids in Water," in Water Chlorination:
Chemistry, Environmental Impact, and Health Effects, Volume 6, Jolley et
al., eds., in press.
Jensen, J.J., J.D. Johnson, J. St.Aubin and R.F. Christman (1985)
"Effect of Monochloramine on Isolated Fulvic Acid", Organic
Geochem., 8(1)71-76.
Jensen, J.J. et al., (1985) "Characterization of the Reaction
Between Monochloramine and Isolated Aquatic Fulvic Acid", Water
Chlorination: Environmental Impact and Health Effects, Vol 5, R.L. Jolley
et al., Eds., Lewis Publ. Chelsea, MI.
Jestin, J.M., Y. Levi & J. Hotelier (1986) Proc. Journees
Information Eaux, Univ. de Poitiers, France, Conf #3.
Kool, H.J., J. Hrubec, C.F. Van Kreijl & G.J. Piet (1985) The
Science of the Total Environment, 47:229.
Kool, H.J., C.F. Van Kreijl & H. Van Oers (1984) Toxicol. &
Environ. Chem., 7:111.
Kopfler, F.C., R.G. Melton, R.D. Lingg & W. Emile Coleman (1976) Identification
& Analysis of Organic Pollutants in Water, L.H. Keith ed., Ann Arbor
Sci. Publ., Ann Arbor, MI, p87
Kopfler, F.C., H.P. Ringhand, W.E. Coleman & J.R. Meier (1985) Water
Chlorination: Chemistry, Environmental Impact and Health Effects, Vol.5,
Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p161.
Kringstad, K.P., F. de Sousa & L.M. Stromberg (1985) Envir. Sci.
& Technol., 19(5)427.
Kringstad, K.P., P.O. Ljungquist, J. de Sousa & L.M. Stromberg (1983)
Environ. Sci. & Technol., 17(8)468.
Kronberg, L., B. Holmbom & L. Tikkanen (1986) Aquatic
Micropollutants in the Environment, Bjorseth & Angeletti, eds., D.
Reidel Publ., Boston.
Kronberg, L. and T. Vartianinen (1988) "Ames Mutagenicity and
Concentration of the Strong Mutagen
3-Chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone and of its Geometric
Isomer E-2-chloro-3-(dichloromethyl)-4-oxo-butenoic Acid in Chlorine Treated Tap
Waters," In press.
Landrum, P.F., S. R. Nihart, B.J. Eadie adn W.S. Gardner
(1984) "Reverse-Phase Separation Method for Determining Pollutant Binding
to Aldrich Humic Acid and Dissolved Organic Carbon of Natural Waters", Environ.
Sci. & Technol., 18(3)187-192.
Larson, R.A. (1978)
"Dissolved Orgnanic Matter of a Low-colored Stream, Freshwater Biology,
8:91-104.
Meier, J.R. & R.J. Bull (1985) Water Chlorination: Chemistry,
Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis
Publ., Inc., Chelsea, MI, p207.
Meier, J.R., Lingg, R.D.
& R.J. Bull (1983) Mut. Res., 118:25.
Merlet, N. (1986) "Contribution a l'Etude du Mecanisme de Formation
des Trihalomethanes et des Composes Organohalogenes Non-Volatils lors de la
Chloration de Molecules Modeles", Doctoral Thesis, University of Poitiers,
France
Minear, R.A. (1981) Proceedings of the International Conference,
"Water Industry", Brighton, UK, pp.105-113.
Minisci, J.R. and R. Galli (1965) Tetrahedron Lett., 1965(8)433.
Moore, H.E., M.J. Garmendia
& W.J. Cooper (1984) Environ. Sci. & Technol., 18(5)348.
Morris, J.C. (1967) in Principles and Applications of Water Chemistry,
S.D. Faust & J.V. Hunter, eds., John Wiley publ., New York.
Morris, J.C. & B. Baum (1978) Water Chlorination: Environmental
Impact and Health Effects, Vol.2, R.L. Jolley ed., Ann Arbor Sci. Publ.,
Ann Arbor, MI., p29.
Nazar, M.A. & W.H.
Rapson (1982) Envir. Mut., 4:435.
Norwood, D.L., R.F. Christman, J.D. Johnson & J.R. Hass (1986) J.
Am. Wat. Wrks. Assn., 78(4)175.
Norwood, D.L., J.D. Johnson, R.F. Christman & D.S. Millington (1983) Water
Chlorination: Environmental Impact and Health Effects, Vol.4, Jolley et
al., Eds., Ann Arbor Sci. Publ., Inc., Ann Arbor, MI, p191.
NRC (1980) Drinking
Water and Health, Volume 2, National Academy Press, Wash.
NRC (1987) Drinking
Water and Health, Volume 7, National Academy Press, Wash.
Oliver, B.G. (1983) Envir.
Sci. & Technol., 17(2)80.
Qinn, J.E. & V.L.
Snoeyink (1980) J. Am. Wat. Wrks. Assn., 72(8)483.
Rav-Acha, Ch., E. Chosen
and B. Limoni (1983) J. Envir. Sci. Hlth., A18.
Randtke, S.J. and C.P.
Jepsen (1981) J. Am. Wat. Wrks. Assn., 73(8)411-419.
Reckhow, D.A. (1984) Ph.D. Dissertation, University of North
Carolina, Chapel Hill, NC
Reckhow, D.A., Bezbarua,
B., Bose, P., and A. MacNeill (1993) report to US EPA, RREL
Reckhow, D.A. and P.C.
Singer (1984) J. Am. Wat. Wrks. Assn., 76(4)151-157.
Reckhow, D.A. & P.C. Singer (1985) Water Chlorination: Chemistry,
Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis
Publ., Inc., Chelsea, MI, p1229.
Reckhow, D.A., P.C.
Singer and R.L. Malcolm (1990) Environ. Sci. Technol. 24(11)1655-1664)
Rook, J.J. (1977) Environ.
Sci. & Technol., 11(5)478.
Sato, T., M. Mukaida, Y. Ose, H. Nagase & T. Ishikawa (1985) The
Sci. of the Totl Envir., 46;229.
Schnitzer, M. and S.U.
Khan (1978) Soil Organic Matter, Elsevier, Amsterdam.
Scully, F.E., J.P. Yang, K. Mazina & F.B. Daniel (1984) Environ.
Sci. & Technol., 18(10)787.
Seeger, D.R., L.A. Moore & A.A. Stevens (1985) Water Chlorination:
Chemistry, Environmental Impact and Health Effects, Vol.5, Jolley et al.,
Eds., Lewis Publ., Inc., Chelsea, MI, p859.
Shank, R.C. and C. Whittaker (1988) "Formation of Genotixic
Hydrazine by the Chloramination of Drinking Water," University of Califorina
Water Resources Center, Technical Completion Report, Project No. W-690.
Shiraishi, H., N.H. Polkington, A. Otsuke & K. Fuwa (1985) Environ.
Sci. & Technol., 19(7)585.
Singer, P.C., J.J. Barry III, G.M. Palen and A.E. Scrivner
(1982) "Trihalomethane Formation in North Carolina Drinking Waters", J.
Am. Water Works Assn., 73:392-401.
Stevens, A.A., R.C. Dressman, R.K. Sorrell & H.J. Brass (1985) J.
Am. Wat. Wrks. Assn., 77(4)146.
Trehy, M.L. & T.I. Bieber (1981) Advances in the Identification
& Analysis of Organic Pollutants in Water, Volume 2, L.H. Keith, ed.,
Ann Arbor Sci., Chapt 48, pg 941-975.
Werdehoff, K.S. & P.C. Singer (1986) AWWA Annual Conference
Proceedings, AWWA, Denver, CO, p347.
Wilcox, P. & S. Denny (1985) Water Chlorination: Chemistry,
Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis
Publ., Inc., Chelsea, MI, p1341.
Colclough, C.A. (1981)
"Organic Reaction Products of Chlorine Dioxide and Natural Aquatic Fulvic
Acids", MS Thesis, Univ. of North Carolina, Chapel Hill, NC.
Coleman, W.E., J.W.
Munch, W.H. Kaylor, R.P. Streicher, H.P. Ringhand & J.R. Meier (1984) Environ.
Sci. & Technol., 18(9)674.
Coleman, W.E., J.W.
Munch, R.P. Streicher & P.A. Hodakievic (1986) 192nd ACS Mtg, Division
of Environmental Chem. Preprints, ACS, Washington, p332.
Condie, L.W. & J.P.
Bercz (1985) Water Chlorination: Chemistry, Environmental Impact and Health
Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p345.
Croue, J.-P. (1987)
"Contribution a l'Etude de l'Oxydation par le Chlore et l'Ozone d'Acides
Fulviques Naturels Extraits d'Eaux de Surface", Doctoral Thesis,
University of Poitiers, France.
de Leer, E.W.B., J.S.S.
Damste, C. Erkelens & L. de Galan (1985) Envir. Sci. & Technol.,
19(6)512.
Holmbom, B., L.
Kronberg, P. Backlund, V.-A. Langvik, J. Hemming, M. Reunanen, A. Smeds &
L. Tikkanen (1987) presented at the Sixth Conference on Water Chlorination, Oak
Ridge, TN.
Holmbom, B., R.H. Voss,
R.D. Mortimer & A. Wong (1984) Environ. Sci. & Technol.,
18(5)333.
Jensen, J.J., J.D.
Johnson, J. St.Aubin and R.F. Christman (1985) "Effect of Monochloramine
on Isolated Fulvic Acid", Organic Geochem., 8(1)71-76.
Jensen, J.J. et al.,
(1985) "Characterization of the Reaction Between Monochloramine and Isolated
Aquatic Fulvic Acid", Water Chlorination: Environmental Impact and
Health Effects, Vol 5, R.L. Jolley et al., Eds., Lewis Publ. Chelsea, MI.
Jestin, J.M., Y. Levi
& J. Hotelier (1986) Proc. Journees Information Eaux, Univ. de
Poitiers, France, Conf #3.
Kool, H.J., J. Hrubec,
C.F. Van Kreijl & G.J. Piet (1985) The Science of the Total Environment,
47:229.
Kool, H.J., C.F. Van
Kreijl & H. Van Oers (1984) Toxicol. & Environ. Chem., 7:111.
Kopfler, F.C., R.G.
Melton, R.D. Lingg & W. Emile Coleman (1976) Identification &
Analysis of Organic Pollutants in Water, L.H. Keith ed., Ann Arbor Sci.
Publ., Ann Arbor, MI, p87
Kopfler, F.C., H.P.
Ringhand, W.E. Coleman & J.R. Meier (1985) Water Chlorination:
Chemistry, Environmental Impact and Health Effects, Vol.5, Jolley et al.,
Eds., Lewis Publ., Inc., Chelsea, MI, p161.
Kringstad, K.P., F. de
Sousa & L.M. Stromberg (1985) Envir. Sci. & Technol., 19(5)427.
Kringstad, K.P., P.O.
Ljungquist, J. de Sousa & L.M. Stromberg (1983) Environ. Sci. &
Technol., 17(8)468.
Kronberg, L., B. Holmbom
& L. Tikkanen (1986) Aquatic Micropollutants in the Environment,
Bjorseth & Angeletti, eds., D. Reidel Publ., Boston.
Meier, J.R. & R.J.
Bull (1985) Water Chlorination: Chemistry, Environmental Impact and Health
Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p207.
Meier, J.R., Lingg, R.D. & R.J. Bull (1983) Mut. Res.,
118:25.
Merlet, N. (1986)
"Contribution a l'Etude du Mecanisme de Formation des Trihalomethanes et
des Composes Organohalogenes Non-Volatils lors de la Chloration de Molecules
Modeles", Doctoral Thesis, University of Poitiers, France
Minear, R.A. (1981)
Proceedings of the International Conference, "Water Industry",
Brighton, UK, pp.105-113.
Moore, H.E., M.J.
Garmendia & W.J. Cooper (1984) Environ. Sci. & Technol.,
18(5)348.
Morris, J.C. & B.
Baum (1978) Water Chlorination: Environmental Impact and Health Effects,
Vol.2, R.L. Jolley ed., Ann Arbor Sci. Publ., Ann Arbor, MI., p29.
Nazar, M.A. & W.H. Rapson (1982) Envir. Mut.,
4:435.
Norwood, D.L., R.F.
Christman, J.D. Johnson & J.R. Hass (1986) J. Am. Wat. Wrks. Assn.,
78(4)175.
Norwood, D.L., J.D.
Johnson, R.F. Christman & D.S. Millington (1983) Water Chlorination:
Environmental Impact and Health Effects, Vol.4, Jolley et al., Eds., Ann
Arbor Sci. Publ., Inc., Ann Arbor, MI, p191.
NRC (1980) Drinking Water and Health, Volume 2,
National Academy Press, Wash.
NRC (1987) Drinking Water and Health, Volume 7,
National Academy Press, Wash.
Oliver, B.G. (1983) Envir. Sci. & Technol.,
17(2)80.
Qinn, J.E. & V.L. Snoeyink (1980) J. Am. Wat. Wrks.
Assn., 72(8)483.
Reckhow, D.A. (1984)
Ph.D. Dissertation, University of North Carolina, Department of Environmental
Science & Engineering.
Reckhow, D.A. & P.C. Singer (1984) J. Am. Wat. Wrks.
Assn., 76(4)151.
Reckhow, D.A. & P.C.
Singer (1985) Water Chlorination: Chemistry, Environmental Impact and Health
Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p1229.
Rook, J.J. (1977) Environ. Sci. & Technol.,
11(5)478.
Sato, T., M. Mukaida, Y.
Ose, H. Nagase & T. Ishikawa (1985) The Sci. of the Totl Envir.,
46;229.
Scully, F.E., J.P. Yang,
K. Mazina & F.B. Daniel (1984) Environ. Sci. & Technol.,
18(10)787.
Seeger, D.R., L.A. Moore
& A.A. Stevens (1985) Water Chlorination: Chemistry, Environmental
Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc.,
Chelsea, MI, p859.
Shiraishi, H., N.H.
Polkington, A. Otsuke & K. Fuwa (1985) Environ. Sci. & Technol.,
19(7)585.
Stevens, A.A., R.C.
Dressman, R.K. Sorrell & H.J. Brass (1985) J. Am. Wat. Wrks. Assn.,
77(4)146.
Trehy, M.L. & T.I.
Bieber (1981) Advances in the Identification & Analysis of Organic
Pollutants in Water, Volume 2, L.H. Keith, ed., Ann Arbor Sci., Chapt 48, pg 941-975.
Werdehoff, K.S. &
P.C. Singer (1986) AWWA Annual Conference Proceedings, AWWA, Denver, CO,
p347.
Wilcox, P. & S.
Denny (1985) Water Chlorination: Chemistry, Environmental Impact and Health
Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p1341.