CHAPTER VI
CHEMICAL CHARACTERISTICS OF NATURAL WATERS
A. INTRODUCTION
The vast majority of the world's natural water is in the oceans. Only about 2½% is in the form of fresh water (Table 6.1). Of this fresh water, fully two-thirds is locked in glaciers, permanent snow cover and permafrost. The remaining 30-31% is largely groundwater. In fact groundwater probably accounts for 98-99% of the earth's liquid-phase water. The rest is mostly in lakes (0.85%), soil moisture (0.16%), atmospheric moisture (0.13%) swamps (0.10%), rivers (0.02%), and in biological tissues (0.01%).
Table 6.1
The World's Water Reserves
(adapted from Shiklomanov, 1993)
|
Percentage of Global Reserves |
||||
|
Volume (103 km2) |
of Total Water |
of Fresh Water |
||
|
World Ocean |
1,338,000 |
96.5 |
||
|
Groundwater Saline Groundwater Fresh Groundwater Soil Moisture |
23,400 12,850 10,530 16.5 |
1.7 0.94 0.76 0.001 |
30.1 0.05 |
|
|
Glaciers & Permanent Snow Antarctic Greenland Arctic Islands Mountainous Regions |
24,064 21,600 2,340 83.5 40.6 |
1.74 1.56 0.17 0.006 0.003 |
68.7 61.7 6.68 0.24 0.12 |
|
|
Ground ice/Permafrost |
300 |
0.022 |
0.86 |
|
|
Lake Water Fresh Saline |
176.4 91 85.4 |
0.13 0.007 0.006 |
0.26 |
|
|
Swamp Water |
11.47 |
0.0008 |
0.03 |
|
|
River Water |
2.12 |
0.0002 |
0.006 |
|
|
Biological Water |
1.12 |
0.0001 |
0.003 |
|
|
Atmospheric Water |
12.9 |
0.001 |
0.04 |
|
|
Total Water |
1,386,000 |
100 |
||
|
Total Fresh Water |
35,030 |
2.53 |
100 |
|
B. INORGANIC MATRIX
1. Occurrence
Because of their slow movement, groundwaters are often thought of being in equilibrium with their surrounding minerals. For example, this often seems to be the case when the controlling phase is calcite. River water may be thought of as a mixture of base-flow from groundwater infiltration plus runoff from precipitation events. Althougth the runoff will have accumulated some additional solutes in its journey across the watershed (from soil, plants, etc), it will still be lower in ionic strength than the base-flow groundwater. Lakes offer a longer residence time, and many reactions that begin in rivers and streams, progress much further in lakes. Of particular importance is the growth of planckton, and the accompanying exchange of oxygen, inorganic nutrients (C, N, P) and biochemicals (sugars, saccharides, proteins, etc.).
The chemical equilibrium processes that give rise to groundwaters, and to a lesser extent, surface waters, involve dissolution of minerals and reaction with dissolved carbon dioxide. An excellent source of information on these processes is in the water chemistry texts of Stumm and Morgan (1981), Snoeyink and Jenkins (1980), and Pankow (1991). Issues related specifically to groundwater quality are covered in Freeze and Cherry (1979). Lake water quality is dealt with by Wetzel (1975).
Although its difficult to generalize about concentrations, a few extensive surveys have been published. Table 6.2 shows the results of a rew of these.
Table 6.2
Average Concentration of Major Inorganic Species in River Water
(values in mg/L)
|
Species |
Global Avg. |
N. America Median |
Colorado Ave. |
|
Ca+2 |
13.4 |
40 |
83 |
|
Mg+2 |
3.3 |
10 |
24 |
|
Na+ |
5.1 |
31 |
95 |
|
K+ |
1.3 |
2.4 |
5.0 |
|
Al+3 |
0.01 |
||
|
Cl- |
5.7 |
16.5 |
82 |
|
F- |
0.17 |
||
|
Br- |
0.019 |
||
|
SO4-2 |
8.3 |
33 |
270 |
|
HCO3- |
52 |
225 |
135 |
|
SiO2 |
10.4 |
16.5 |
9.3 |
|
Total |
100 |
330 |
703 |
2. Specific Solutes
a. pH
The pH of a water is a measure of its hydrogen ion concentration (H+). Quantitatively, pH is the negative of the logarithm of the hydrogen ion activity (equation 6.1). Even in a perfectly-pure water containing no other solutes, a small fraction of the H2O molecules will break-up or dissociate, forming H+ and OH-. The degree to which this occurs is determined by the equilibrium constant for water, which is given the special symbol, Kw. The full equilibrium quotient is shown in Equation 6.2 (see also Chapter 5).
![]() (6.1)
(6.1)
![]() (6.2)
(6.2)
In dilute systems, the activity of pure substances does not change. By convention this activity is assigned a value of one, so equation (6.2) becomes:
![]() (6.3)
(6.3)
In pure water, there will be an equal concentration of H+ and OH-. Therefore, the concentration of each must be 10-7M, and the pH is 7 (at 25°C). When acids are added to a water, {H+} goes up, and the pH drops. When bases are added {H+} goes down, and the pH increases. For this reason, pH values above 7 are said to be alkaline (or basic), and pH values below 7 are said to be acidic. Because the value for Kw is increases with temperature, so does the hydrogen ion activity. At 30°C, pure water has a pH of 6.92, whereas at 0°C it is 7.48.
The pH is a water quality variable of primary importance. It not only defines the acid/base quality of a water, but it determines how the water will react with chemical substances with which it comes into contact. It also defines whether a water is too caustic or corrosive to support biological growth.
The pH of natural waters are generally different from what one would expect for pure water. Natural waters are commonly exposed to acids (e.g., carbon dioxide) and bases (e.g., calcite minerals) which can substantially alter the equilibrium pH. Most groundwaters have pHs between 6.0 and 8.5. River waters are more commonly in the range of 6.5 to 8.5. Some highly alkaline waters may have pHs of 9 or higher. On the other side of the scale, alpine lake waters may have pHs as low as 4.5, and acid mine drainage waters may be much lower still. For more on pH refer to Chapter XVIII.
b. Electrical Conductivity
Conductivity is a measure of the ability of a water to conduct electricity. This conduction of electricity occurs by migration of dissolved ions. Since the more ions present the greater electrical current, conductivity may be though of a measure of the ionic strength. It is also closely related to the total dissolved solids, because most solutes in natural waters are ionic when in a dissolved state.
Conductivity is widely measured in natural waters, partly because it is so easy to do. It can also be readily monitored by passing a water through a continuously-reading conductivity cell. Most natural waters have conductivities in the range of 50-50,000 µmho/cm. The higher limit is approximately the conductivity of sea water. In groundwaters, conductivity tends to increase in the direction of flow. This can provide some useful flow history data.
c. Oxygen
Oxygen occurs in natural waters in a dissolved state. Unpolluted waters that are in good contact with the atmosphere, become rapidly saturated with oxygen. This may produce levels as high as 14 mg/L in cold waters. Surface waters may contain concentrations in the range of 7-12 mg/L. Groundwaters can run the full range from 0 to 12 mg/L. Groundwaters from recharge areas with little or no soil, and permeable, fractured rock may have dissolved oxygen (D.O.) concentrations near the upper end of this range. Those shallow groundwaters from areas with sandy or gravelly soils will have lower, but detectable D.O. levels (i.e., >0.1 mg/L). Those from areas silty or clayey soils may be below 0.1 mg/L. Deep aquifers will have waters with low D.O. regardless of the nature of the recharge area.
d. Silica
Silicon is widely present in natural waters. It comes from the dissolution of quartz and related silicate minerals. Silicon is the second most abundant element in the earth's crust. Once dissolved, the silica becomes hydrated to form hydrosilicic acid (H4SiO4). Because there are only a small number of solid minerals that control its solubility, silicon concentrations in natural waters are relatively uniform, typically 1-30 mg/L as SiO2. Waters in the South and Western US tend to be toward the high end of this range. Some groundwaters may be as high as 100 mg/L. Near-surface sea water may be much lower due to rapid utilization by diatoms.
e. Aluminum
Aluminum is widely present in the hydrosphere (3rd in abundance to oxgen and silicon). Because of its facile hydrolysis to form aluminum hydroxide, aluminum concentrations are generally low.
f. Carbonates
Carbonates are acquired through absorption of atmospheric carbon dioxide and subsequent reaction with bases to form bicarbonate and even some carbonate. Bicarbonate is one of the first inorganic solutes that a groundwater picks up as it moves along its flow path. The carbonates are the major buffering ions in most natural waters. They define the pH of waters and the resistence to change in pH (e.g., from acid rain).
g. Sulfate
Sulfate is the second major inorganic solutes that a groundwater picks up as it moves along its flow path. Although rain water may exhibit elevated sulfate concentrations due to absorption of sulfur oxides, most aqueous sulfate comes from minerals such as gypsum and pyrite.
h. Chloride
Chloride is the third major inorganic solutes that a groundwater picks up as it moves along its flow path. Especially old groundwaters have high chloride levels, and thus they may resemble sea water.
C. ORGANIC MATRIX
The organic matrix of natural waters is dominated by naturally-occurring organic compounds (NOM or natural organic matter). Only in the most exceptional cases do man-made or anthropogenic pollutants predominate. Although its not a pollutant in the classic sense, NOM is of great concern to environmental engineers because it:
1. QUANTITY OF ORGANIC MATTER FOUND IN NATURAL WATERS
Organic compounds are present in all natural waters as both dissolved organic carbon (DOC) and particulate organic carbon (PtOC). The distinction between particulate and dissolved is truly an operational one. Typically, samples are filtered in the laboratory through a glass fiber filter disk (0.45m m or 1.2m m nominal pore size). Organic matter that passes through is counted as dissolved and that which is retained is particulate. Particulate organic carbon may include bacteria, phytoplankton, zooplankton and organic-coated minerals (Figure 1). The dissolved forms tend to be non-living organic molecules and macromolecules such as the humic materials, lignins, tannins, carbohydrates, amino acids, proteins, fatty acids and many others. In this discussion we will focus on the dissolved forms. The DOC is important in understanding the mineral-associated POC, as the dissolved compounds form the pool of organic matter from which organic coatings on mineral particles are derived. This discussion will also only cover the naturally occurring organic compounds. For information on anthropogenic compounds the reader is referred to Keith (1977 & 1981).
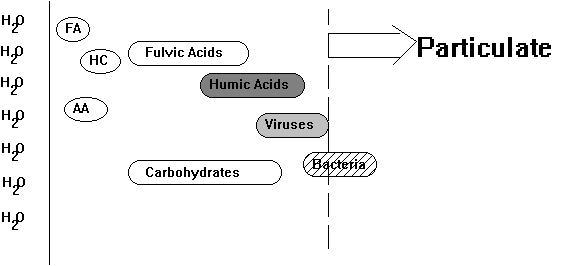
Figure 6.1
Relative Size of Natural Organic Carbon
FA=fatty acids; CHO=carbohydrates; AA=amino acids; HC=hydrocarbons
(Modified from: Thurman, 1985)
On the average 2-3% of the suspended solids in a river or lake water is composed of particulate organic carbon. As TSS goes up, the organic fraction commonly decreases. Nevertheless, gross POC concentrations tend to increase with increasing suspended solids concentration. The median worldwide POC concentration for river water has been estimated at 2.5 mg/L (Meybeck, 1981; 1983). In river and lake waters the DOC concentrations are determined by a balance of formation through primary production (photosynthetic plants) and loss due to biodegradation. Tropical rivers often have higher DOC concentrations than rivers of similar size in temperate climates due to greater primary production rates. Riverine systems showing a strong seasonal flow pattern generally show the same pattern for DOC. That is, carbon concentrations increase with increasing flow. Furthermore, DOCs are generally higher on the ascending portion of the hydrograph. As one might expect, large rivers tend to have higher DOCs than small streams.
In lakes, DOC is a strong function of the trophic state. As lakes increase in productivity (i.e., algal activity), they also show an increase in DOC. The dystrophic lakes receive heavy loads of humic materials from marshes and bogs which explains their extremely high DOC. In lakes, the POC usually amounts to about 10% of the DOC.
Table 6.3
Dissolved Organic Carbon Concentration in Lakes (mg/L)
(after Thurman, 1985)
|
Trophic State |
Mean DOC |
Range |
|
Oligotrophic |
2 |
1-3 |
|
Mesotrophic |
3 |
2-4 |
|
Eutrophic |
10 |
3-34 |
|
Dystrophic |
30 |
20-50 |
Most groundwaters have DOC's of less than 2 mg/L and insignificant POC concentrations. The median US groundwater DOC is thought to be about 0.7 mg/L (Leenheer et al., 1974). Interstitial soil waters have much higher DOC's (2-30 mg/L) which decrease quickly with depth to concentrations typical of those in groundwater. Similarly, interstitial sediment water DOC concentrations are quite high, ranging from 4-20 mg/L under aerobic conditions and 10-400 mg/L under anaerobic conditions. In contrast, the DOC of rainfall is only about 1 mg/L, but it may quickly rise to 5 or 10 mg/L upon contact with the forest canopy. The mean concentration of DOC in seawater is 0.5 mg/L below 300 meters and 1.0 mg/L above.
2. TYPES OF ORGANIC MATTER FOUND IN NATURAL WATERS
Natural organic matter is extremely diverse. At best, we are only able to identify about 20% of the DOC (Buffle, 1988). While the complete identification of DOC may never be possible, we can always categorize this material according to physico-chemical properties or similarities in chemical structure.
A. ANALYTICALLY DEFINED GROUPS
1. Organic Carbon Fractions
A comprehensive fractionation scheme (Leenheer, 1981), recently developed for the preparative isolation of natural organic matter, will be presented as a basis for group classifications. A sample is first passed through an adsorption column filled with XAD-8 non-ionic resin. This adsorbant removes all of the hydrophobic materials. The effluent, containing only hydrophilic compounds, is sequentially passed through a cation exchange column followed by an anion exchange column. The hydrophilic bases are retained by the cation exchange column and the hydrophilic acids end up on the anion exchange column. The hydrophilic neutrals pass through all three columns untouched.
Each fraction must be separately eluted from their respective adsorbents and purified. The hydrophobic bases are eluted from the XAD column with 0.1 N hydrochloric acid, and the hydrophobic acids are removed with 0.1N sodium hydroxide. In order to isolate hydrophobic neutrals, the resin must be removed and extracted with methanol. Each of the ion exchange columns may be eluted with ammonium hydroxide. Hydrophilic bases are obtained from the cation exchange column, and hydrophilic acids elute from the anion exchange column. The final column effluent, containing only hydrophilic neutrals, is isolated by vacuum rotary evaporation.
The fractionation scheme of Leenheer is useful in part, because it is comprehensive. Thus, it allows us to account for nearly all of the organic carbon in natural waters even though we don't know the identity of much of this material. Figure 2 shows a typical break-down expressed as percent dissolved organic carbon
2. Humic Substances
Humic substances are a group of highly-colored polyfunctional organic acids. Since their structure is unknown, they are most commonly defined by the extraction procedure used to isolate them. That is, they are organic materials that are removed by XAD-8 acrylic ester resin at pH 2 and eluted at pH 13. Of these, the fulvic acids are the soluble organics at pH 2, and any organics that precipitate out at this pH are called humic acids.
The concentrations of humic substances are quite variable depending on the water source. Their overall contribution to the DOC tends to be directly proportional to the absolute value of the DOC. Thus, for uncolored waters (e.g., an oligotrophic lake) the humic substances may account for 25% of the total carbon, whereas highly-colored waters (e.g., from wetlands) may be as much as 90% humic. Usually 85% of the humic substances are fulvic acids and the remaining 15% are humic acids. However, the ratio of humic acids to fulvic acids also tends to increase with increasing DOC. There are also important seasonal variations in the concentrations of humic substances. Commonly, fulvic acid concentrations will follow overall DOC concentrations.
Riverine humic substances are generally of allochthonous origin. These organics originate from sources outside of the water body, such as soil and terrestrial plants. Lacustrine humic substances may be both of allochthonous and autochthonous in origin (n.b.: autochthonous means originating from within the water body). The autochthonous sources, primarily algae, become more important as the lake becomes larger.
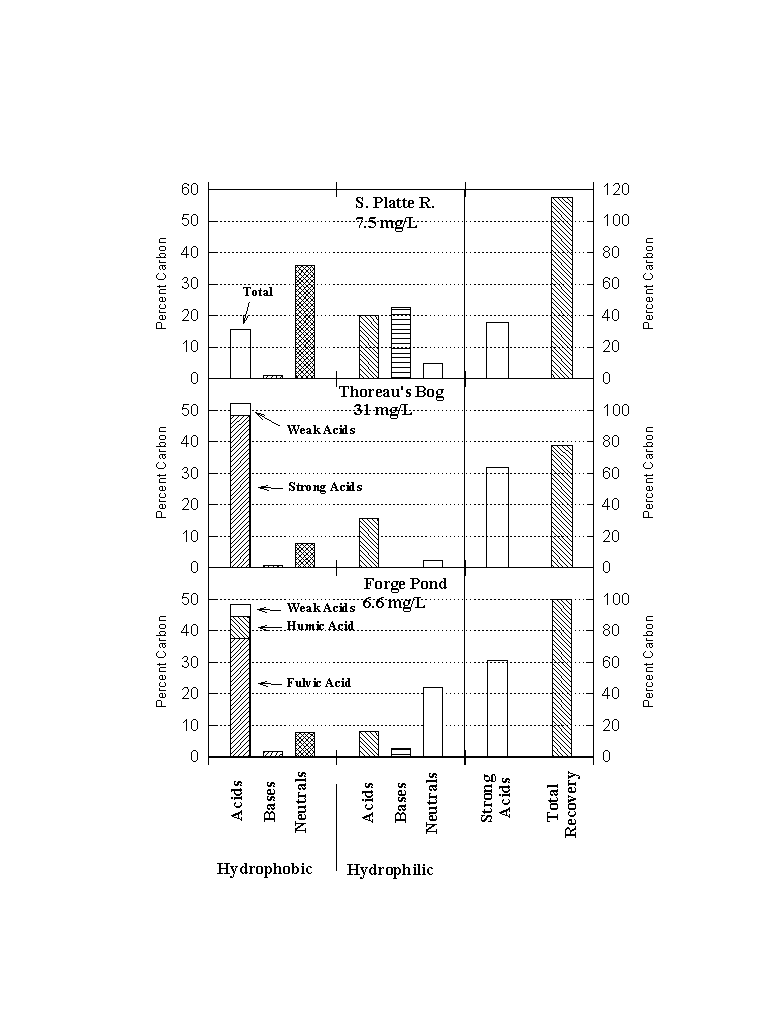 Figure 6.2
Figure 6.2
Natural Organic Matter Fractions From Three Surface Waters
(S. Platte R. from Leenheer, 1981; Thoreau's Bog from McKnight et al., 1985; Forge Pond from Reckhow et al., 1993)
3. Hydrophilic Acids
This is another operationally-defined group. They are sometimes defined as the organic acids not retained by XAD resin at pH 2. The hydrophilic acids are difficult to isolate and analyze, therefore, little is known about them. Leenheer (1981) believes these include hydroxyacids, polyacids, polyhydroxyacids and some low MW fatty acids. They may also encompass the sugar acids such as uronic, aldonic and polyuronic acid (Thurman, 1985). For this discussion we will include here all non-nitrogenous organic acids of primarily aliphatic nature except the simple fatty acids.
B. STRUCTURALLY DEFINED GROUPS
1. Tannins, Aromatic Acids, and Phenols
Tannins are plant products made up of gallic acid monomers. They may be either hydrolyzable or condensed (i.e., flavenoids). Due to analytical ambiguities, little is known about the concentrations of these compounds. An educated guess would place them at about 1% of the DOC.
Aromatic acids and phenols may account for 0.5% of the total DOC although more data are needed to make a firm conclusion (Thurman, 1985). It is believed that these are products of the degradation of lignin and tannins. Indeed, most of the identified aromatic acids strongly resemble lignin monomers.
2. Carbohydrates
Carbohydrates are sugars or sugar polymers. Such compounds are essentially polyhydroxy aldehydes. Single sugars (e.g., glucose) are called monosaccharides and are thought to account for about 1% of the DOC in natural waters. The most common of these is glucose. Oligosaccharides which contain 10 simple sugars or less, and polysaccharides containing more than 10 sugar units, amount to about 5% of the total DOC. Humic sugars which are bound to humic materials make up about 2%. Other variations containing additional functional groups (1% of the DOC) are the sugar acids, amino sugars and sugar alcohols.
Alginic acid, a polysaccharide made from D-mannuronic acid, is commonly used in Europe as a coagulant aid. This compound is obtained commercially from a number of algal species. Not surprisingly, most carbohydrates found in lake water arise from phytoplankton. Their concentrations increase with increasing trophic level (i.e., algal populations). However, most riverine carbohydrates originate from terrestrial plants. Roughly 30% of the mass of terrestrial flora ends up in the aquatic environment, and more than half of this material is carbohydrate in nature. Most of the carbohydrates can be found in the hydrophilic neutral fraction when using Leenheer's comprehensive fractionation.
3. Fatty Acids
Fatty acids are biologically active compounds whose concentrations are subject to seasonal variations. They are generally divided into two distinct groups: volatile (about 4% of the DOC) and nonvolatile (approximately 2% of the DOC). Acetic acid is the predominant volatile fatty acid although many others are commonly found (see figure 3). These acids have properties which class them with the hydrophilic acids. Acetic and other volatile fatty acids arise primarily from the microbial degradation of natural organic matter. Butyric acid and hexanoic acid, byproducts of blue-green algae, are suspected of being sources of tastes and odors in certain waters. Concentrations of volatile fatty acids are often inversely correlated with the oxidation potential and dissolved oxygen concentration of a water, and positively correlated with sulfide concentrations.
H-COOH CH3-COOH CH3-CH2-COOH CH3-CH2-CH2-COOH
Formic Acid Acetic Acid Propionic Acid Butyric Acid
CH3-CH2-CH2-CH2-COOH
Valeric Acid
Figure 3
Common Volatile Fatty Acids in Natural Waters
Palmitic acid and stearic acid are the most common of the nonvolatile acids (see Figure 4). The corresponding unsaturated acids tend to be less common, perhaps because they are more rapidly degraded. Also, the acids with even numbers of carbon atoms predominate. These probably come from the hydrolysis of lipids derived from algae and terrestrial plants. Since algae are roughly 15% fat, they represents a significant source.
CH3-(CH2)12-COOH CH3-(CH2)16-COOH CH3-(CH2)18-COOH
Myristic Acid Palmitic Acid Stearic Acid
Figure 4
Common Nonvolatile Fatty Acids in Natural Waters
4. Amino Acids and Proteins
This well studied fraction is composed of the free amino acids (0.5 % of the DOC), the humic-bound amino acids (0.75% of the DOC), and proteins & polypeptides (2% of the DOC). Of the free amino acids, glycine and serine are particularly prominent. Amino acid distributions are highly dependent on the nature of the surrounding flora, and thus they may be geographically quite specific. There are indications that the concentrations of these compounds in rivers increases as the temperature decreases. In lakes their concentrations are closely tied to the algal populations; increasing as the algae die and lyse. Furthermore, amino acids are generally a more significant fraction of the DOC in lakes (especially eutrophic lakes) than in other waters. The amino acids and proteins can be found in the hydrophilic and hydrophobic base fractions as shown in Figure 2.
Amino acids and proteins may be preferentially adsorbed on to mineral particles. This would be expected based on the predominance of net negative surface charges on the particles at neutral pH, and the positive charges of amino groups. Indeed the nitrogen/carbon ratio in suspended solids has been observed to be greater than for the surrounding bulk soil. Because of their complexity, little is known about the combined forms of amino acids in water. Proteins and polypeptides have been found in water with molecular weights exceeding 5000 amu. These compounds may have an especially large tendency to associate with particle surfaces, because of their high molecular weight.
5. Hydrocarbons
In unpolluted waters, hydrocarbons amount to less than 1% of the overall DOC. These naturally-occurring hydrocarbons include the saturated hydrocarbons (alkanes and isopreniods), unsaturated hydrocarbons (alkenes), saturated cyclic hydrocarbons, unsaturated cyclic hydrocarbons, and simple and fused ring aromatic hydrocarbons (polynuclear aromatic hydrocarbons). Although the most common alkane is methane, many other semi-volatile species can be found in water. Most of these are chains of 15-33 carbons. Since alkanes are commonly biosynthesized from the decarboxylation (i.e., loss of COOH) of fatty acids, they are dominated by species containing odd numbers of carbon atoms. This is the case, because the naturally occurring fatty acids generally contain even numbers of carbon atoms. The branched alkanes include the isoprenoids found in phytoplankton, zooplankton and bacteria. Among the naturally occurring polynuclear aromatic hydrocarbons (PAHs) are 3,4-benzopyrene, retene, and perylene.
6. Miscellaneous Low MW Compounds
Several dicarboxylic acids have been isolated in natural waters. It has been estimated that they amount to about 0.5% of the DOC. Figure 5 shows the structure of some of these compounds, several of which are important biochemical intermediates (e.g., TCA cycle).
HOOC-COOH HOOC-CH2-COOH HOOC-CH2-CH2-COOH
Oxalic Acid Malonic Acid Succinic Acid
Figure 5
Dicarboxylic Acids Found in Natural Waters
The hydroxy and keto acids are important biochemical intermediates (e.g., glycolysis). They are rapidly degraded in the aquatic environment, and therefore, their concentrations are low (~1% of the DOC). Little is known about these compounds, because they are difficult to isolate. These are found in the hydrophilic acid fraction.
Aldehydes, both aliphatic and aromatic, are another group found in natural waters. Although their total concentrations are quite small, they may form a major portion of the readily volatile organic carbon in unpolluted waters. Sterols, a type of isoprenoids, are also found at low concentrations. These compounds are found in natural waters, because they are used in the construction of cell membranes, and as regulators of various cellular functions. A well know example is cholesterol.
Among the miscellaneous compounds are a number of volatile and semi-volatile algal byproducts that are responsible for many of the naturally-occurring tastes and odors in drinking water. The best known are Geosmin (trans-1,10-dimethyl-trans-9-decalol) and 2-Methyliosborneol or just MIB (1,2,7,7-tetramethyl-exo-bicyclo(2.2.1)heptan-2-ol) which have been attributed "earthy-musty" odors. These are produced by many species of Actinomycetes, a widespread group of filamentous bacteria, and some Blue-green algae (Mallevialle & Suffet, 1987). Other important odor-producing byproducts of the Actinomycetes include cadin-4-ene-1-ol ("woody-earthy") and 2-isopropyl-3-methoxy-pyrazine (IPMP, a "potato bin" odor). The aldehydes, n-heptanal and n-hexanal have been isolated from cultures of various types of algae. They apparently have strong "fishy" odors.
Certain amines may be important sources of fishy odors in drinking water. Most of these are degradation products from algal amino acids. Examples include the aminopropanes, ethanolamine, butanolamines, putrescine, cadaverine, tyramine, tryptamine, and phenylethylamine. Organic bases of the type incorporated in DNA and RNA are presumed to be present in all natural waters. These include the purines (i.e., adenine and guanine) and the pyrimidines (i.e., thymine, cytosine and uracil). Adenine is also present in the form of adenosine triphosphate (ATP) and adenosine diphosphate (ADP). These are highly correlated with active microbial growth. Since ATP has three strongly ionized phosphate groups it would be isolated along with the hydrophilic acid group. Most organic bases that are known biochemical products have not been found in natural water, presumably because they are cationic and will readily associate with sediments by ion exchange. They may, however, be important in determining the behavior of suspended sediments. One exception is urea, the principle form of nitrogen excreted by animals. Since it is largely unionized at neutral pHs, it has been found in natural waters in concentrations as high as 30 ug/l (Larson, 1978). The porphyrins, including the chlorophyll species, are important plant pigments. These are relatively insoluble nitrogeneous compounds. Therefore, they are present almost entirely as algal particulate organic carbon.
Much of the organic phosphorus in natural waters is thought to be present in the form of inositol (a six carbon monosaccharide) phosphates and phosphates of glucose and fructose. The concentrations of these forms are probably controlled by calcium, aluminum and iron. These sugar phosphates are thought to form insoluble complexes with Ca, Al and Fe much the way orthophosphate does. Therefore, highly colored humic waters which contain low concentrations of polyvalent cations may have high concentrations of sugar phosphates. Organic sulfur compounds are well known for their algal origins and tendency to impart strong odors to natural waters (Jenkins et al., 1967). Among the greatest offenders from this group are dimethylsulfide and methyl sulfide.
LITERATURE CITED
Babcock, D.B. and P.C. Singer (1979) J. Am. Wat. Wrks. Assn., 71(3)149.
Buffle, J. (1988) Complexation Reactions in Aquatic Systems: An Analytical Approach, John Wiley & Sons, publ., New York.
Burttschell, R.H., A.A. Rosen, F.M. Middleton & M.B. Etinger (1959) J. Am. Wat. Wrks. Assn., 51(2)205.
Carter, C.W. and I.H. Suffet (1982) "Binding of DDT to Dissolved Humic Materials", Environ. Sci.& Technol., 16:735-740.
Carter, C.W. and I.H. Suffet (1985) "Quantitative Measurements of Pollutant Binding to Dissolved Humic Materials with Bulk Properties of Humic Materials", Org. Geochem., 8(1)145-146.
Chiou, C.T., P.E. Porter and D.W. Schmedding (1983) "Partition Equilibria of Nonionic Organic Compounds Between Soil Organic Matter and Water", Environ. Sci & Technol., 17(4)227-231.
Crochet, R.A. and P. Kovacic (1973) J. Chem. Soc. Commun., 19:716.
Croue, J.-P. (1987) "Contribution a l'Etude de l'Oxydation par le Chlore et l'Ozone d'Acides Fulviques Naturels Extraits d'Eaux de Surface", Doctoral Thesis, University of Poitiers, France.
Davies, S.M. and R.J.M. De Wiest (1966) Hydrogeology, Wiley, New York.
De Haan, H., T. De Boer, H.A. Kramer and J. Voerman (1982) Water Res., 16;1047-1050.
Dempsey, B.A. and C.R. O'Melia (1983) Aquatic and Terrestrial Humic Materials, pp 239-273, Christman & Gjessing editors, Ann Arbor Science, Ann Arbor, MI
Dempsey, B.A., R.M. Ganho and C.R. O'Melia (1984) J. Am. Wat. Wrks. Assn., 76(4)141-150.
Edzwald, J.K., W.C. Becker and K.L. Wattier (1985) "Surrogate Prarmeters for Monitoring Organic Matter and THM Precursors", J. Am. Water Works Assn., 77(4)122-132.
Freeze, R.A. and J.A. Cherry (1979) Groundwater, Prentice-Hall, Inc., Englewood Cliffs, NJ.
Gauthier, T.D., W.R. Seitz and C.L. Grant (1987) Environ. Sci. & Technol., 21(3)243-252.
Gjessing, E.T. and L. Berglind (1981) "Adsorption of PAH to Aquatic Humus," Arch. Hydrobiol., 1:92:24.
Hall, E.S. and R.F. Packham (1965) J. Am. Wat. Wrks. Assn., 57(9)1149-1166.
Handa, N. (1966) "Examination on the Applicability of the Phenol Sulfuric Acid Method on the Determination of Dissolved Carbohydrate in Sea Water, J. of the Oceanographic Soc. of Japan, 22:81-86.
Jenkins, D., L.L. Medsker, and J.F. Thomas (1967) "Odorous compounds in Natural Waters. Some Sulfur Compounds Associated with Blue-Green Algae, Environ. Sci. & Technol., 1:731-735.
Jestin, J.M., Y. Levi & J. Hotelier (1986) Proc. Journees Information Eaux, Univ. de Poitiers, France, Conf #3.
Kool, H.J., J. Hrubec, C.F. Van Kreijl & G.J. Piet (1985) The Science of the Total Environment, 47:229.
Kool, H.J., C.F. Van Kreijl & H. Van Oers (1984) Toxicol. & Environ. Chem., 7:111.
Kopfler, F.C., R.G. Melton, R.D. Lingg & W. Emile Coleman (1976) Identification & Analysis of Organic Pollutants in Water, L.H. Keith ed., Ann Arbor Sci. Publ., Ann Arbor, MI, p87
Kringstad, K.P., F. de Sousa & L.M. Stromberg (1985) Envir. Sci. & Technol., 19(5)427.
Kringstad, K.P., P.O. Ljungquist, J. de Sousa & L.M. Stromberg (1983) Environ. Sci. & Technol., 17(8)468.
Landrum, P.F., S. R. Nihart, B.J. Eadie adn W.S. Gardner (1984) "Reverse-Phase Separation Method for Determining Pollutant Binding to Aldrich Humic Acid and Dissolved Organic Carbon of Natural Waters", Environ. Sci. & Technol., 18(3)187-192.
Larson, R.A. (1978) "Dissolved Orgnanic Matter of a Low-colored Stream, Freshwater Biology, 8:91-104.
Leenheer, J.A. (1981) Environ. Sci. & Technol., 15(5)578-587.
Leenheer, J.A. (1985) Humic Substances in Soil, Sediment, and Water: Geochemistry, Isolation, and Characterization, pp 409-429, Aiken et al., eds., John Wiley & Sons Publ., New York.
Mallevialle, J. and I.H. Suffet (1987) Identification and Treatment of Tastes and Odors in Drinking Water, Cooperative Research Report, AWWA Research Foundation, Denver, CO
Meybeck, M. (1979) "Concentrations des Eaux Fluviales en Elements Majeurs et Apports en Solution aux Oceans," Revue Geogr. Phys. 21:215-246.
Meybeck, M. (1981) "Pathways of Major Elements from Land to Ocean Through Rivers", in River Inputs to Ocean Systems, J.M. Martin, J.B. Burton and D. Eisma, Eds., UN Press, New York.
Minisci, J.R. and R. Galli (1965) Tetrahedron Lett., 1965(8)433.
Moore, H.E., M.J. Garmendia & W.J. Cooper (1984) Environ. Sci. & Technol., 18(5)348.
Nazar, M.A. & W.H. Rapson (1982) Envir. Mut., 4:435.
Pankow, J.F. (1991) Aquatic Chemistry Concepts, Lewis Publ., Chelsea, MI.
Rav-Acha, Ch., E. Chosen and B. Limoni (1983) J. Envir. Sci. Hlth., A18.
Randtke, S.J. and C.P. Jepsen (1981) J. Am. Wat. Wrks. Assn., 73(8)411-419.
Reckhow, D.A. (1984) Ph.D. Dissertation, University of North Carolina, Chapel Hill, NC
Reckhow, D.A., Bezbarua, B., Bose, P., and A. MacNeill (1993) report to US EPA, RREL
Reckhow, D.A. and P.C. Singer (1984) J. Am. Wat. Wrks. Assn., 76(4)151-157.
Reckhow, D.A. & P.C. Singer (1985) Water Chlorination: Chemistry, Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p1229.
Reckhow, D.A., P.C. Singer and R.L. Malcolm (1990) Environ. Sci. Technol. 24(11)1655-1664)
Sato, T., M. Mukaida, Y. Ose, H. Nagase & T. Ishikawa (1985) The Sci. of the Totl Envir., 46;229.
Schnitzer, M. and S.U. Khan (1978) Soil Organic Matter, Elsevier, Amsterdam.
Scully, F.E., J.P. Yang, K. Mazina & F.B. Daniel (1984) Environ. Sci. & Technol., 18(10)787.
Semmens, M.J. and A.B. Staples (1986) J. Am. Wat. Wrks. Assn., 78(2)76-81.
Shank, R.C. and C. Whittaker (1988) "Formation of Genotoxic Hydrazine by the Chloramination of Drinking Water," University of Califorina Water Resources Center, Technical Completion Report, Project No. W-690.
Shiklomanov, I.A. (1993) "World Fresh Water Resources," in Water in Crisis: A Guide to the World's Fresh Water Resources, P.H. Gleick ed., Oxford University Press, New York, pp.13-24.
Shiraishi, H., N.H. Polkington, A. Otsuke & K. Fuwa (1985) Environ. Sci. & Technol., 19(7)585.
Sinsabaugh, R.L. III, R.C. Hoehn, W.R. Knocke and A.E. Linkins III (1986a) J. Am. Wat. Wrks. Assn., 78(5)74-82.
Sinsabaugh, R.L. III, R.C. Hoehn, W.R. Knocke and A.E. Linkins III (1986b) ASCE J. of Environ. Engin., 112(1)139-153.
Smart, P.L., B.L. Finkiyson, W.D. Rylands and C.M. Ball (1976) "The Relation of Fluorescence to Dissolved Organic Carbon in Surface Waters", Water Res., 10:805-811
Snoeyink, V.L. and D. Jenkins (1980) Water Chemistry, J. Wiley & Sons, New York.
Stevens, A.A., R.C. Dressman, R.K. Sorrell & H.J. Brass (1985) J. Am. Wat. Wrks. Assn., 77(4)146.
Stuum, W. and J.J. Morgan (1981) Aquatic Chemistry, J. Wiley & Sons., New York.
Thurman, E.M. (1985) Organic Geochemistry of Natural Waters, Nijhoff & Junk Publ., Boston.
Thurman, E.M. and R.L. Malcolm (1979) "Concentration and Fractionation of Hydrophobic Organic Acid Constituents from Natural Waters by Liquid Chromatography" U.S. Geological Survey Water-Supply Paper 1817-G.
Van Breemen, A.N., Th.J. Nieuwstad and G.C. Van der Meent-Olieman (1979) Wat. Res., 13(8)771-779.
Vik, E.A., D.A. Carlson, A.S. Eikum and E.T. Gjessing (1985) "Removing Aquatic Humus from Norwegian Lakes," J. Am. Wat. Wrks. Assn., 77(3)58-66.
Werdehoff, K.S. & P.C. Singer (1986) AWWA Annual Conference Proceedings, AWWA, Denver, CO, p347.
Wetzel, R.G. (1975) Limnology, W.B. Saunders Co., Philadelphia.
Wilcox, P. & S. Denny (1985) Water Chlorination: Chemistry, Environmental Impact and Health Effects, Vol.5, Jolley et al., Eds., Lewis Publ., Inc., Chelsea, MI, p1341.