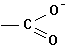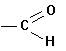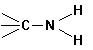CHAPTER
III
ORGANIC
NOMENCLATURE
Until
recently, the environmental engineer did not have to be very knowledgeable
regarding organic chemistry, and the naming of organic compounds
(nomenclature). However, the
intensifying concern over anthropogenic organic pollutants has changed all of
this. Now the environmental
professional must be familiar with the basic terminology of organic
chemistry. He/she must also have a
certain knowledge of the chemical behavior of organic compounds as it relates
to environmental processes. This
chapter is intended to provide a working knowledge of organic nomenclature.
A. THE CARBON SKELETON
Carbon
can form a nearly limitless diversity of compounds. One reason for this is carbon's ability to bind covalently with
itself in long chains:
![]()
In the above structure, each carbon atom
(C) is surrounded by four single bonds.
This is a consequence of carbon's tendency to form four covalent bonds
each. These extra bonds not used to
join the carbon chain may be linked to hydrogen atoms or other structures. The particular structure shown above is an aliphatic
chain. The carbons are linked in a
linear fashion, without forming rings or cycles.
1. Unbranched Alkanes
An
homologous series of simple aliphatic organic compounds is then the following:
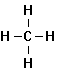
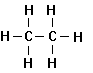
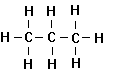
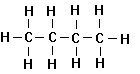
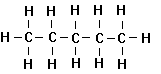
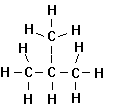
Methane Ethane Propane Butane
Pentane
The series continues: hexane (6C),
heptane (7C), octane (8C), nonane (9C), decane (10C), etc. This is the simplest homologous series of
organic compounds, called the normal alkanes or sometimes the normal paraffins. Alkanes are saturated hydrocarbons
(containing only hydrogen and carbon) with an unbranched aliphatic
structure. To emphasize their simple
linear structure they are often given the prefix, "n-", meaning,
normal. All alkanes have the general
empirical formula, CnH2n+2.
2. Branched Alkanes & IUPAC
Nomenclature
Branched
alkanes are also commonly encountered.
From a geometric standpoint, the smallest branched aliphatic has 4
carbons. It is called Isobutane because
it is an isomer of butane (often referred to n-butane to distinguish it from
isobutane). An isomer is a
compound with an empirical formula identical to a second compound, but with a
different structure (i.e., geometric arrangement of the atoms) is
different. For this reason, branched
alkanes are sometimes called isoalkanes or isoparaffins.
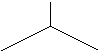
Full Structure
Shorthand
Isobutane
In
order to avoid the tedium and unnecessary detail of drawing in all of the
hydrogen atoms, chemists have adopted a shorthand version of representing
organic structures. The rules of this
shorthand are as follows. No carbon
atoms belonging to the carbon skeleton are drawn. Instead, only the bonds connecting these carbon atoms are
shown. It is understood that a carbon
atom is present at the junction of any two or more line segments. In addition, the lines, representing bonds,
are generally drawn at a slight angle to each other (often ~120°). This is often close to the actual bond
angles, and it makes the separation of one carbon-carbon bond from the next
more obvious. When single bonds connect
the carbon atoms, a single line is used, and when double or triple bonds exist,
double or triple lines are used.
Hydrogen atoms connected directly to the carbon skeleton and the bonds
that connect them are never shown.
Instead their presence can always be determined by keeping in mind that
all carbon atoms must have a total of four covalent bonds. It is understood that bonds not shown (those
required to reach a total of 4 per carbon) are actually carbon-hydrogen
linkages, and that a hydrogen atom is at the end of each of these bonds.
Returning
to the branched alkanes, there are three possible isomers of pentane (saturated
5C hydrocarbons). Add one more carbon
and the number increases to five. At
ten carbons, there exists 75 possible isomers.
Since we can't refer to all of the branched isomers as simply isoheptane
or isodecane (the unbranched alkanes are always n-heptane, n-decane, etc.) a
well defined system of naming these compounds is needed. The International Union of Pure and Applied
Chemistry (IUPAC) has developed just such a system. For example, consider the following isomers of heptane.
![]()
![]()
![]()
![]()
![]()
n-Heptane 2-Methylhexane 3-Methylhexane 3-Methylhexane 2-Methylhexane
The first structure is a simple alkane
with 7 carbons (i.e., n-Heptane). The
next four have seven carbons, but they are all branched. According to the IUPAC rules, aliphatic
compounds are named after the longest continuous chain of carbon atoms that can
be found in the structure. For all four
of these, the longest component chain is 6 carbon, thus they are all hexane
derivatives. Also in each case, the
branch is composed of a single carbon atom.
Thus, they are all termed, methylhexanes. The name methyl comes from meth-, signifying one carbon atom
(e.g., methane). Instead of adding the
-ane suffix indicating an alkane, -yl is added, which indicates an alkyl chain
(i.e., alkane minus a hydrogen). The
precise names for the methylhexanes are unambiguously assigned once the
geometric location of the methyl branch is determined. To do this, the carbon atoms on the alkane
backbone are numbered consecutively from one end to the other. The number of the carbon atom to which the
methyl branch is bound identifies the particular methylhexane isomer. Note that the last two are not called
4-methylhexane and 5-methylhexane. This
is because, these structures are identical to 3-methylhexane and 2-methylhexane
respectively. One can see this by just
flipping them over.
3. Alkenes
If
one were to remove two hydrogens from each of the alkanes, leaving a
carbon-carbon double bond in their place, one would have the series known as alkenes
or olefins. Organic compounds
such as these having double or triple bonds are often referred to as unsaturated,
because they have less than the maximum possible number of hydrogens. The IUPAC names for these compounds have the
same basic roots as the alkanes, however, the suffix -ene is used in place of
-ane. Common usage also permits the
suffix "-ylene" for alkenes, especially for ethene (i.e.,
ethylene). Unbranched alkenes of 4
carbons or greater can have isomers, depending on the location of the double
bond. The system of numbering the
carbons on the carbon backbone, and assigning the position of the double bond
to the lowest number of the two carbons involved. When more than one double bond is present the suffix becomes
-diene, -triene, etc. All alkenes with
only one double bond have the general empirical formula, CnH2n.
![]()
![]()
Ethene Propene 1-Butene 2-Butene 1-Pentene 2-Pentene
![]()
3-Ethyl-5-Octene 1,3-Pentadiene
4. Alkynes
Removal
of 4 hydrogens from two adjoining carbons in an alkane results in the formation
of a carbon-carbon triple bond. The
homologous series of these compounds is termed the alkynes (suffix
-yne). Despite the IUPAC nomenclature,
the first member of this series is most commonly known as acetylene. All alkynes with only one triple bond have
the general empirical formula, CnH2n-2.
![]()
![]()
Ethyne Propyne 1-Butyne 2-Butyne 1-Pentyne 2-Pentyne
5. Alicyclic Hydrocarbons
When
hydrocarbon chains are joined to make a ring, they are said to be cyclic, or
more properly, alicyclic. These
differ from the previously discussed aliphatic hydrocarbons which may be
branched, but not in the form of rings.
A compound need only have one ring to be considered alicyclic,
regardless of whether there are aliphatic chains attached to it. They may also have double and triple bonds
(e.g., cycloalkenes, cycloalkynes, in addition to cycloalkanes). However, if a compound contains a 6-membered
ring with alternating double and single bonds, it is a member of a special
class called "the aromatics" (see below).
Alicyclic
compounds are often given the prefix, "cyclo". Alicyclic alkanes are sometimes called naphthenes. These compounds have the general empirical
formula, CnH2n.
Note that this is the same formula for the aliphatic alkenes. Thus, addition of a double-bond or closure
to form a ring have the same effect on a compound's empirical formula.
![]()
![]()
Cyclopentane Cyclohexane
6. Aromatics
Six-membered
rings tend to be quite stable. However,
when a six-membered ring contains three alternating double and single bonds, it
has a property known as resonance. This
imparts a special stability to the molecule, and for this reason we give such
molecules a special name, aromatic.
The simplest aromatic compound is benzene.
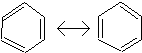 or
or ![]()
Benzene
There
are a wide range of benzene derivatives of great commercial and environmental
importance. These may include the
simple aromatic hydrocarbons, the hydroxy-benzenes or phenols, the biphenyls,
and the fused aromatics otherwise known as polynuclear aromatics.
The
simple aromatic hydrocarbons (below) can help illustrate several important
points. First, notice that when a
single carbon group (a methyl group) is attached to benzene, it is called
toluene. The IUPAC name should be
methylbenzene, but toluene has been used for so long that it is now well
accepted. Similarly, the xylenes are
actually dimethylbenzene isomers. These
three isomers are distinguished by the relative positions of the methyl groups. When groups attached to the aromatic ring
(known as substituents) are on adjacent carbons, they are said to be in
the ortho position (represented by
the prefix, "o-"). When they are two carbons away they are in
the meta position ("m-"). And when they are on opposite sides of the ring, they are in the para position ("p-"). This is not only true for xylenes, but for any aromatic compound
with multiple substituents.
![]()
![]()
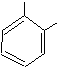
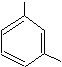
![]()
Toluene Ethylbenzene o-Xylene m-Xylene p-Xylene
When
3 or more substituents are present, the numbering system is most commonly
used. One of the six carbons is
assigned the number "1", and the others are numbered consecutively as
you go clockwise or counterclockwise around the ring. The direction you choose to go around the ring doesn't matter,
because an aromatic compound can always be flipped on its vertical axis to get
the mirror image. These two forms are
indistinguishable and represent the same compound.
Example: Automotive Gasoline:
Gasoline is a blend of light
hydrocarbon fractions from petroleum.
It is blended at the refinery with the very practical objective to
perform well in combustion engines.
Since, its not blended to any specific chemical composition, its precise
makeup is hard to characterize. Of all
the major petroleum products, gasoline probably has the highest alkane content,
a low alicyclic content, and a low to medium aromatic content. It differs from jet fuel in that the latter
is higher in alicyclics and lower in alkanes.
The fuel oils have higher aromatic contents, average alicyclic contents
and very low levels of alkanes.
Table 3.1 shows the composition
of two contrasting automotive gasolines.
The 76 product is a winter-grade (volatility class D) regular fuel. The Amoco is a summer-grade (volatility
class A) premium fuel. The winter-grade
fuel (76) is characterized by more volatile constituents. This is especially seen in the higher alkane
content. The premium (Amoco) has a
higher octane rating and a substantially higher toluene concentration.
Efficient operation of automobile
engines requires that just the right mix of hydrocarbon vapor and air is
obtained in the carburator. If the
hydrocarbon vapor concentration is too high, there will be too little oxygen
for good combustion. Conversely, if the
vapor concentration is too low, ignition will not occur at all. For this reason, manufacturers blend their
gasoline to achieve the right volatility for the anticipated temperture
conditions (i.e., location and time of year).
High octane gasolines are blended
to minimize knocking, or irregular ignition.
As a hydrocarbon becomes more heavily branched, it tends to ignite more
smoothly in an automobile engine. One
of the most efficient compounds in this regard is 2,2,4-trimethylpentane[1], which is arbitrarily given an octane
rating of 100. n-Heptane, on the other
hand, is very poor, and is given a rating of zero. Aromatics have high octane ratings of 100 or more. This is why the high-aromatic content Amoco
is a "premium grade".
In practice, octane ratings are
determined empirically by comparing the performance of a gasoline with standard
mixtures of n-heptane and 2,2,4-trimethylpentane. Two reading are generally taken, one on a cold engine (the R or
research rating), the other on warm engine (the M or motor rating). The overall octane rating is then taken as
the average of the two, i.e., ![]()
In 1922 it was discovered that
the octane rating of a gasoline could be increased by addition of small amounts
of tetraethyl lead. This compound has a
central lead atom surrounded by four ethyl groups. It resembles a highly-branched alkane and greatly inhibits
knocking. For the next 50 years leaded
gasoline was the norm, until congress required all new car to have catalytic
converters. Since tetraethyl lead
poisoned the catalyst, unleaded gasoline was needed. To help achieve the desired no-knock performance, octane-boosting
additives, such as methyl tert-butyl
ehter (MTBE; 115 octane rating), tert-butyl
alcohol and methyl alcohol (105 octane rating), are now used.
Table 3.1
Composition of Two Unleaded Gasolines
(from: Sigsby et al., 1987)
|
Compound |
% by Weight |
|
|
|
76 Regular |
Amoco Premium |
|
n-Alkanes |
|
|
|
n-Butane |
7.75 |
3.52 |
|
n-Pentane |
3.06 |
2.37 |
|
n-Hexane |
1.32 |
0.83 |
|
n-Heptane |
1.23 |
0.42 |
|
n-Octane |
0.76 |
0.20 |
|
n-Nonane |
0.27 |
0.18 |
|
Branched
Alkanes |
|
|
|
Isobutane |
1.86 |
1.4 |
|
2,2-Dimethylbutane |
0.41 |
0.08 |
|
2,3-Dimethylbutane |
0.86 |
0.78 |
|
Isopentane |
6.16 |
7.12 |
|
2-Methylpentane |
2.76 |
2.76 |
|
3-Methylpentane |
1.76 |
1.47 |
|
2,4-Dimethylpentane |
1.15 |
0.86 |
Table 3.1 (continued)
Composition of Two Unleaded Gasolines
(from: Sigsby et al., 1987)
|
Compound |
% by Weight |
|
|
|
76 Regular |
Amoco Premium |
|
Branched
Alkanes (cont) |
|
|
|
2,3,3-Trimethylpentane |
2.26 |
1.82 |
|
3-Methylhexane |
1.91 |
1.04 |
|
2,3,5-Trimethylhexane |
0.18 |
0.13 |
|
2,2,5-Trimethylhexane |
0.81 |
0.76 |
|
2-Methylheptane |
0.37 |
0.10 |
|
3-Methylheptane |
0.70 |
0.23 |
|
4-Methylheptane |
1.20 |
0.25 |
|
3,4-Dimethyloctane |
1.12 |
1.42 |
|
2-Methyldecane |
1.83 |
1.38 |
|
Alkenes |
|
|
|
2-Butene |
0.50 |
0.26 |
|
1-Pentene |
0.32 |
0.18 |
|
2-Pentene |
1.40 |
1.14 |
|
1-Hexene |
0.64 |
0.64 |
|
2-Hexene |
0.33 |
0.27 |
|
3-Hexene |
0.80 |
0.73 |
|
Branched
Alkenes |
|
|
|
2-Methyl-2-butene |
1.22 |
1.50 |
|
2-Methyl-2-pentene |
0.61 |
0.65 |
|
Dimethylhexene |
0.28 |
0.25 |
|
Cyclic
Compounds |
|
|
|
Cyclopentene |
0.37 |
0.31 |
|
Cyclopentane |
0.48 |
0.42 |
|
Methylcyclopentane |
1.17 |
0.77 |
|
Cyclohexene |
2.73 |
1.31 |
|
Methylcyclohexane |
1.57 |
0.33 |
|
Aromatics |
|
|
|
Benzene |
1.76 |
1.96 |
|
Toluene |
5.54 |
20.25 |
|
Ethylbenzene |
1.17 |
0.94 |
|
m- and p-Xylene |
4.58 |
2.60 |
|
o-Xylene |
2.46 |
1.61 |
|
n-Propylbenzene |
0.70 |
0.90 |
|
1,3,5-Trimethylbenzene |
2.74 |
3.35 |
|
1,2,4-Trimethylbenzene |
3.75 |
4.59 |
|
1,2,3-Trimethylbenzene |
1.21 |
1.26 |
|
1-Methyl-3-ethylbenzene |
1.52 |
1.53 |
|
Isobutylbenzene |
0.42 |
0.48 |
|
1-Methyl-3-n-propylbenzene |
1.15 |
1.32 |
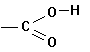
B. FUNCTIONAL GROUPS
In
addition to hydrocarbon chains, one can build organic compounds by adding what
we call functional groups. These are
common sub-molecules or structures that contain an "O", an
"N" an "S" or a
variety of other elements, in addition to carbon and/or hydrogen. Some examples follow:
|
|
|
Primary |
Secondary |
|
Name |
Structure |
Suffix/Prefix |
Term |
|
Alcohols |
|
-ol |
hydroxy |
|
Acids |
|
-oic
acid |
carboxy |
|
|
|
-ate |
|
|
Ketones |
|
-one |
keto |
|
Aldehydes |
|
-al |
|
|
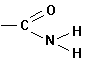
Amines |
|
-yl
amine |
amino |
|
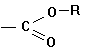
Esters |
|
Alkyl(R)
__-ate |
|
|
Ethers |
|
|
|
|
Halides |
|
chloride |
chloro- |
|
|
|
bromide |
bromo- |
|
Amide |
|
|
|
|
Nitriles |
|
-nitrile |
|
C. HETEROATOMS and HETEROCYCLICS
Heteroatoms often refer to nitrogen,
oxygen, sulfur that are incorporated into ringed molecules, called
heterocycles. Thre are a broad array of
heterocyclic compounds one finds in nature, notably the nucleic acids base
units, pyrimidines and purines. Some
important ones are shown below
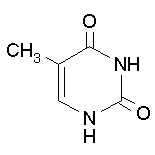
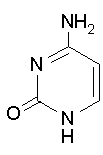
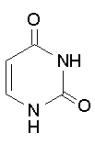
Thymine Cytosine Uracil
Pyrimidines
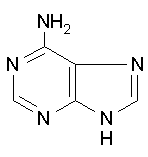
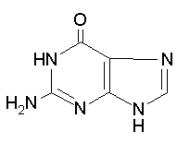
Adenine
Guanine
Purines
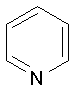
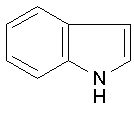
Pyridine Indole
Others
Literature Cited
Sigsby, J.E., S. Tejada, W. Ray, J.M.
Lang and W. Duncan, 1987. "Volatile Organic Compound Emissions from 46
In-Use Passanger Cars," Environ. Sci. Technol. 21(5)466-475.
General References
Sawyer, C.N., P.L. McCarty and G.F.
Parkin, 1994. Chemistry for Environmental Engineering, 4th Edition,
McGraw-Hill, Inc., New York. (chapter 5)
Hutchinson, E. 1964. Chemistry: The
Elements and their Reactions, 2nd Edition, W.B. Saunders Co., Philadelphia,
(chapter 29), or almost any other basic
text on general chemistry or organic chemistry