CHAPTER X
OVERVIEW OF ENVIRONMENTAL ANALYSIS
A. INTRODUCTION
1. Environmental Analysis
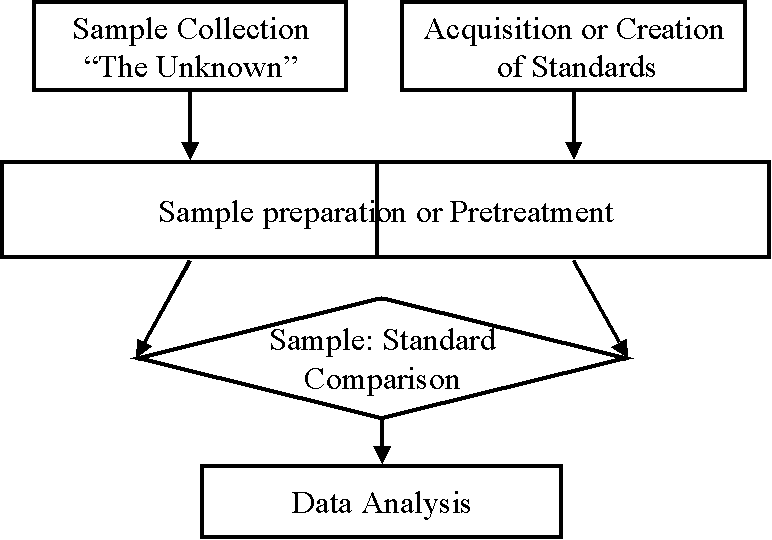
Environmental analysis, as used in these notes, is the chemical (or physical) characterization of some component of the natural or engineered environment. One may speak of four facets to environmental analysis:
1. Analytical Methods
2. Sampling Protocol
3. Quality Control
4. Data Analysis
The first, analytical methods, is what one most commonly associates with chemical analysis of the environment. It is the recipe or set of laboratory procedures one follows in order to obtain a signal that can be related to the concentration of the analyte. Analytical methods are presented in Chapters XV-XXII, and grouped according to the instrument or apparatus used. The second facet, sampling protocol, is sometimes trivial, yet sometimes very important and complex. Sampling is the means by which a subset of the environmental matrix of interest is selected, stored and transported to the laboratory for analysis (Chapter XIII). Then, quality control, is a set of procedures which are intended to warn the analyst when his/her analytical method is not working properly (Chapter XII). Some of these procedures may be implicitly writted into the analytical method. Finally, data analysis, is required to place the analytical results into the framework of a numerical value with uncertainty. Statistical theory plays an important role in this process, and this subject is treated in Chapter XXIII.
2. Analytical Methods
All analytical methods require the comparison of an unknown sample to some type of standard substance or solution. In most cases the standard is chemically identical to the analyte. The key to a good analytical method is to get the analyte into a form that can readily undergo this comparison, and to make the comparison with great accuracy. The first step in this process is sample preparation. Samples must be manipulated prior to comparison with a standard in order to: (1) remove interfering substances; (2) improve the sensitivity and selectivity of the device being used to make the comparison; and (3) to improve the stability of the analyte, or to stabilize the stoichiometry of the reaction used to compare sample with standard. While sample preparation is explicitly addressed in analytical methodology, there is a tendency to present methodology according to the method of comparison used. Therefore, in order to present a clearer and more structured discussion of sample preparation, a separate chapter (XIV) is devoted to this purpose. The second step in chemical analysis is the preparation of a standard. In most cases, this is a trivial step in terms of complexity or level of effort. However, it is of critical importance for the accurate determination of analyte concentration. Finally, the last step is the comparison. This involves the use of some type of instrument or apparatus. When an analytical balance is used, the method is termed gravimetric (Chapter XV). When a buret is used, the method is called volumetric or titrimetric (Chapter XVI). Other instruments that may be used include a spectrophotometer (Chapter XVII), a variety of electrochemical devices (Chapter XVIII), gas chromatograph (Chapter XIX), a liquid chromatograph (Chapter XX), a mass spectrometer (Chapter XXI), and a number of specialized instruments (Chapter XXII).

The following two sections in this chapter attempt to provide a logical framework in which to place the hundreds of analytical methods that may be used in the analysis of aqueous environmental samples. The purpose of this presentation is to provide the reader with a perspective on the diversity of methods and some of the common threads that tie them together. It is of little or no use to memorize the details in the remainder of this chapter. However, these lists may help the student of environmental analysis visualize the full range of methods that one can find in a work such as Standard Methods for the Examination of Water and Wastewater. Many of these methods will be discussed in greater detail in the chapters that follow.
B. TRADITIONAL CLASSIFICATION OF ANALYTICAL METHODS
I. Classical
A. Gravimetric
1. Evaporation
2. Filtration
3. Precipitation
4. Extraction
B. Volumetric Analysis or Titrimetric Analysis
1. Acid/Base
2. Precipitation
3. Complexation or Chelation
4. Oxidation/Reduction
II. Modern or Instrumental Methods
A. Spectroscopic Methods
1. Molecular
a. Molecular Absorption
i. Ultraviolet/Visible
ii. Infrared
b. Molecular Emission
2. Atomic
b. Atomic Absorption (AAS)
i. Flame
ii. Furnace
b. Atomic Emission
i. Flame
ii. Plasma
B. Electrochemical Methods
1. Potentiometry
a. Glass Electrode
b. Ion Selective Electrodes (ISE)
2. Amperometry
3. Voltammetry
4. Conductance
C. Chromatographic Methods
1. Planar Chromatography
2. Gas Chromatography
a. Flame Ionization Detection
b. Electron Capture Detection
c. Thermal Conductivity
3. Liquid Chromatography
D. Mass Spectrometry
a. Probe Introduction
b. GC Introduction (GC/MS)
c. LC Introduction (LC/MS)
E. Nuclear Methods
III. Biochemical Methods
A. Whole Organisms
B. Cell Cultures
C. Enzyme Systems
C. CLASSIFICATION OF STANDARD ENVIRONMENTAL METHODS
I. Classical
A. Gravimetric
A series of sample manipulations leads eventually to the weighing of the residue or precipitate on an analytical balance. Considered an absolute method of analysis, not needing standardization (primary method of analysis).
Sources of error
i. non-quantitative transfer, separation
ii. incomplete precipitation
iii.analytical balance - generally not significant
1. Evaporation
-Residue
2. Filtration
-Suspended Solids
-Volatile Suspended Solids (with final pyrolysis)
-Dissolved Solids (with final evaporation)
3. Precipitation
-Mg: (with Diammonium hydrogen phosphate and final pyrolysis)
-Na: (with zinc uranyl acetate)
-Silica: precipitation/ ignition/ volatilization (with HF)
-SO4: precipitation (with Ba)
4. Extraction
-Oil & Grease: extraction into trichlorotrifluoroethane followed by distillation of the solvent
-Surfactants: into ethyl acetate, evaporation
B. Volumetric Analysis or Titrimetric Analysis
Based on the volume of the titrant, added incrimentally, that is required to reach the equivalence point (the place where the reaction reaches stiochiometric completion). The indicator can either be insitu such as phenolphthalein, or it can be external such as a pH-sensitive, glass potentiometric electrode.
1. Acid/Base
- Alkalinity (indicator: methyl orange)
- Acidity (indicator: phenolphthalein)
- N(-III), Macro-Kjeldahl Method: conversion of organic and free ammonia nitrogen to ammonium sulfate using potassium sulfate and mercuric sulfate (digestion) followed by distillation and titration of the resulting ammonium hydroxide .
- Volatile Acids, Distillation Method: distill then titrate with NaOH
2. Precipitation
- Chloride: with Ag (indicator: potassium chromate)
- Chloride, Mercuric Nitrate Method: with Hg (indicator: diphenylcarbazone)
3. Complexation or Chelation
Ethylenediaminetetraacetic Acid (EDTA)- (HOOCCH2)2NHCH2CH2NH(CH2COOH)2 is by far the most widely used
-Ca: EDTA titrimetric method (Eriochrome Blue Black R)
-Hardness: EDTA titrimetric method (Eriochrome Black T)
-CN: complexation with Ag, detection of excess Ag with p-dimethylaminobenzalrhodanine
4. Oxidation/Reduction
-Dissolved Oxygen, Winkler first Mn is oxidized then I, back titrant: sodium thiosulfate (indicator: starch)
-Ca: permanganate titrimetric method(following precipitation with oxalate and filtration) (self indicating)
-Chlorine & ClO2, Iodometric Method: oxidation of iodide to iodine, titration with sodium thiosulfate (indicator: starch)
-SO3, Iodometric Method: titration with iodide/iodate reagent detection of excess I with starch
-Chlorine & ClO2, DPD Ferrous Titrimetric Method: oxidation of DPD (N,N-diethyl-p-phenylenediamine) and back titration with ferrous ammonium sulfate (DPD is indicator)
-COD: oxidation by dichromate, titration of excess oxidant with ferrous ammonium silfate, redox indicator:ferroin (1,10-phenanthroline + ferrous sulfate))
II. Modern or Instrumental Methods
A. Spectroscopic Methods
1. Molecular
a. Molecular Absorption
i. Ultraviolet/visible Spectroscopy
-Color
-UV absorption (254 nm)
-Al: following complexation with Eriochrome cyanine R dye (535 nm)
-Be: following complexation with aluminon (aurintricarboxylic acid ammonium salt)(515 nm)
-Cd: following complexation with dithizone (diphenylthiocarbazone) and extraction into chloroform (518 nm)
-Cr: following complexation with diphenylcarbazide (540 nm) specific for hexavalent form (considered the more toxic) as opposed to trivalent
-Cu: following complexation with neocuproine (2,9,-dimethyl-1,10-phenanthroline) and extraction into a chloroform-methanol mixture (457 nm)
-Cu, Bathocuproine Method: following complexation with bathocuproine disulfonate (484 nm)
-Fe, Phenanthroline Method: following reduction with hydroxylamine (to ferrous) and chelation with 1,10-phenanthroline
-Pb, Dithizone Method: following extraction into carbon tetrachloride and complexation with dithizone (520 nm)
-Mn, Persulfate (or Periodate) Method: following oxidation to permanganate with persulfate (or periodate) (525 nm)
-Hg, Dithizone Method: following extraction into chloroform and complexation by Dithizone (490 nm)
-Ni, Dimethylglyoxime Method: following double extractions into chloroform & water, and complexation with dimethylglyoxime (445 nm)
-K: measurement of excess dichromate (425 nm) following precipitation with sodium cobaltinitrite and oxidation
-Se, Diaminobenzidine Method: following oxidation (to selenate), reduction (to selenite),complexation with diaminobenzidine and extraction into toluene (420 nm)
-Ag, Dithizone Method: complexation with dithizone (462 or 620 nm)
-Zn, Dithizone Method: following extraction into carbon tetrachloride and complexation with dithizone (535 or 620 nm)
-Zn, Zincon Method: following complexation with zincon (620 nm)
-As, Silver Diethyldithiocarbamate Method: complexation preceeded by reduction by zinc (535 nm)
-B, Curcumin Method: (540 nm)
-B, Carmine Method: (585 nm)
-Br (-I): oxidation to hypobromite by chloramine-T followed by bromination of phenol red (590 nm)
-Chlorine & ClO2, DPD colorimetric method: following oxidation of DPD (515 nm)
-CN: following oxidation to CNCl (cyanogen chloride) by Chloramine-T and reaction with pyridine-barbituric acid (578 nm)
-SCN: following complexation with ferric ion (460 nm)
-F, SPADNS Method: reaction with zirconium (570 nm)
-Iodide (or Iodine), Leuco Crystal Violet Method: oxidation to iodine with potassium peroxymonosulfate followed by reaction with LCV (592 nm)
-V, Gallic Acid Method: catalytic effect of V on the rate of oxidation of gallic acid by persulfate, measure rate at 415 nm
-Iodide, Catalytic Reduction Method: of ceric ions by arsenious acid, the remaining ceric ions are then reduced by ferrous ammonium sulfate, ferric ions measured at 510nm
-NH3, Nessletrization Method: reaction with Nessler reagent (KI + HgI2) measure at 410 nm
-NH3, Phenate Method: formation of indophenol by the reaction of ammonia, hypochlorite, and phenol (630 nm)
-NO3, (and NO2), Cadmium reduction method: reduced to nitrite in the presence of Cd, nitrite is then diazotized with sulfanilamide and coupled with N-(1-naphthyl)-ethylenediamine (543 nm)
-NO3, Chromotripic Acid Method: direct reaction to form a yellow product (410 nm)
-PO4, Stannous Chloride Method: formation of molybdophosphoric acid (from ammonium molybdate) and reduction by SnCl2 (690 nm)
-PO4, Ascorbic Acid Method: reaction of ammonium molybdate and potassium antimonyl tartrate with orthophosphate to form phosphomolybdic acid that is subsequently reduced by ascorbic acid (880 nm)
-Silica,Molybdosilicate Method :formation of
molybdosilicic acid upon the addition of ammonium molybdate (410 nm)
-SO4, methylythymol Blue Method: precip[itation with Ba , chelation of excess Ba with methylthymol blue, unchelated agent is measured at 460 nm
-Sulfide, Methylene Blue Method: reaction of sulfide, ferric chloride and dimethyl-p-phenylenedeamine to produce methylene blue (664 nm)
-Phenols, Chloroform extract method: reaction with 4-aminoantipyrine in the presence of potassium ferricyanide, colored antipyrine dye may be extracted in chloroform (460 nm)
-Tannins & Lignins: reduce tungstophosphoric and molybdophosphoric acids to produce a blue color (700 nm)
ii. Infrared Spectroscopy
-Oils & Grease: extraction into trichlorotrifluoroethane followed by comparison of 2930 cm-1 peak (hydrogen stretching region for methylene hydrogens) with standards, avoids volatilization of hydrocarbons seen in the gravimetric method
b. Molecular Emission
i. Fluorescence: more sensitive & selective, but one also gets unwanted side reactions such as quenching and photodecomposition, low precision and accuracy
-Rhodamine B: used in dye studies, concentrations down to 0.01 ppb can be detected
2. Atomic
b. Atomic Absorption (AAS)
i. Flame
-Cd, Ca, Cr, Co, Cu, Fe, Pb Mg, Mn, Ni, Ag, Zn (Ga,Ru,Rh,Pd,Ir,Pt,Au,Tl,Bi,Sb,Te): by direct aspiration into an Air-Acetylene Flame (T=2250° C max). Can also be used for Na, K, and Sr but flame emission is better. For low levels of Cd, Cr, and Pb prior chelation with Ammonium Pyrrolidine Dithiocarbamate and extraction into Methyl Isobutyl Ketone may be necessary.
-Al, Ba, Be, V and Si (B,Ge,Sn,Sc,Ti,Zr,Nb,Mo,Hf,Ta,W,Re,Os,): by direct aspiraion into a Nitrous Oxide Acetylene Flame (T=2955 C max). For low levels of Al and Be prior chelation with 8-Hydroxyquinoline and extraction into Methyl Isobutyl Ketone may be necessary.
-Hg: by cold vapor method (oxidation of Hg species to Hg(II) by permanganate and persulfate then reduction to Hg vapor by stannous chloride.
-As and Se: by aspiration into an Argon-Hydrogen Flame (T=1577° C max) with prior conversion to their hydrides (oxidation by nitric and perchloric acids followed by reduction with stannous chloride)
ii. Furnace: more sensitive due to larger sample; drying ashing and atomization steps
b. Atomic Emission
i. Flame
-Li, Sr, Na, and K
ii. Direct-Current Arc ; often used with photographic film or plate detection (Spectrograph), internal standards are common. Advantages: high sensitivity, many elements simultaneously, Disadvantages: lengthy & difficult procedure, poor precision.
-Ag, Spectrographic Method: with graphite electrodes
iii.Spark: similar to arc techniques,
iv. Plasma: three types; inductively coupled argon plasma, dc argon plasma, and microwave-induced plasmas of both argon and helium
B. Electrochemical Methods
1. Potentiometry: Definition:the measurement of the electromotive force, potential, that is established between electrodes that are immersed in a silution containing ions that can be oxidized or reduced. Potential differences measured by high input-impedance solid-state amplified meters.
a. Glass Electrode
-Chloride: titration with silver nitrite, potentiometric endpoint determination with a glass and silver-silver chloride electrode system.
-pH:
b. Ion Selective Electrodes
-CN, NH3, NO3, F, Hardness
i. solid-state
ii. liquid-junction
iii.gas-sensing
2. Amperometry: Definition; the measurement of a steady-state current in an electrolytic cell which is due to the oxidation or reduction of the analyte species. There may be no applied potential (galvanic cell) or there may be an applied potential. In this later case, a limiting current is established which is proportional to the concentration of the analyte.
-Dissolved Oxygen, membrane electrode method: (no applied potential) zinc anode, inert (gold or platinum) cathode with a plastic membrane.
-Chlorine, membrane electrode method:
-Ozone, membrane electrode method:
-Chlorine, Iodine & ClO2: titration with phenylarsine oxide (potential may or may not be applied, but 250 mV is often used) until no additional drop in current is observed; platinum (rotating) and silver/silver chloride electrodes are most common. Often here a polarograph set at a fixed voltage is used.
3. Voltammetry: Def;measurement of a current of a redox process as a function of an applied potential giving a voltammogram. Advantages; one of the few analytical techniques that provides direct information about the oxidation state, speciation, and degree of complexation of a specific analyte.
a. Polarography:
-Cu, Pb, Ni, and Zn: in a supporting electrolyte of ammonium sulfate and following digestion with sulfuric and nitric acids.
4. Conductance
-Conductivity
C. Chromatographic Methods
1. Planar Chromatography
a. Paper Chromatography
b. Thin-Layer Chromatography
2. Gas Chromatography
a. FID
-Phenols, DAI: carbowax phase
b. ECD
-Organochlorine Pesticides(BHC, lindane, heptachlor, aldrin, heptachlor epoxide, dieldrin, endrin, captan, DDE,DDD, DDT, methoxychlor, endosulfan, dichloran, mirex, pentachloronitrobenzene): OV-201, OV-17, OV-1 are some recommended stationary phases, solvent; diethyl ether in hexane
-Chlorinated Phenoxy Acid Herbicides (2,4-D, silvex, 2,4,5-T): extraction into either, methylation , columns same as with pesticides
-THM: pentane, SP-1000
c. Thermal Conductivity:based on the heat loss of a filament by gaseous thermal conduction, the thermal conductivity of a gas is based on its diffusion rate thus this detector is most sensitive to small molecules
-Digester Gas: useful phases; silica gel, molecular sieve 13X
3. Liquid Chromatography: Types; Adsorption Chromatography, Partition Chromatography, Ion-exchange Chromatography, Exclusion Chromatography,Uses; purification, identification, quantification
a. Conductivity
i.Anion Exchangers : Ion Chromatography: Advantages; it effectively distinguishes among the halides, no hazardous reagents
-Br, Cl, F, SO3 SO4, NO2, NO3, PO4
b. Refractive Index: based on the ability of solvents to deflect light, a universal detector
c. Ultraviolet Absorbance
d. Fluorescence
e. Other
-Volatile Acids: silicic acid column, butanol in chloroform eluant, detection by base titration
D. Mass Spectrometry
a. Probe Introduction
b. GC Introduction (GC/MS)
-Taste & Odor Organics (Geosmin, 2-Methylisoborneol, etc.): concentration by CLSA
-Acidic Oxidation by-products: extraction with ether, methylation
c. LC Introduction (LC/MS)
E. Nuclear Methods
1. Neutron Activitation
F. Miscellaneous and Composite Instruments
a. TOC
-Combustion/IR method: pyrolysis at 950 C, detection of CO2 by IR
-Persulfate/UV method: UV oxidation in the presence of persulfate, detection of CO2 by IR
b. TOX
-Adsorption/Pyrolysis/Titrimetric Method: microcoulometric detection where the amount of halide is quantified by measuring the current produced by silver-ion precipitation of the halides.
III. BIOCHEMICAL METHODS
A. Whole Organism
1. Human
-Taste & Odor
2. Bacteria
-BOD
3. Miscellaneous Aquatic Organisms (Algae, Daphnia, Fish, etc.)
-Acute Toxicity: phytoplankton, zooplankton, daphnia, copepod, coral, annelid worms, crustaceans, aquatic insects, mollusks & fish
-Chronic Toxicity
-Biostimulation (Algal Productivity)
B. Cell Cultures
1. Chinese Hamster
-Ames Test
C. Enzyme Systems
-Specific Organic Electrodes