|
CEE 370 |
Spring 2001 |
Exam #2
April 19, 2001
Closed Book, two sheets of notes allowed
Please answer any 4 of the following 9 questions. Each is worth 25 points. Show all work. Be neat, and box-in your answer.
1. Settling Design (25 points)
You’ve been asked to design a pair of identical primary clarifiers for treating wastewater in the Town of Springfield. The town engineer wants you to size a circular clarifier that is capable of removing particles with a diameter of 0.1 mm and a density of 1.4 g/mL, even in the mid-winter when wastewater temperatures drop to 5 C. If the town’s wastewater flow averages 17 MGD, and it peaks at 25% over this, what diameter clarifier will you recommend? Keep in mind that the town engineer has asked for some specific performance that might not be assured using just the standard design criteria.
Solution:
Use Stoke’s Law:
![]()
considering the temperature 5 C, and the density and viscosity data on the front cover of the text book,
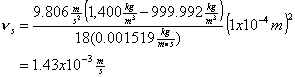
For the purpose of clarifier design, we set the overflow rate to the settling velocity of the particles that we wish to just be able to capture:
![]()
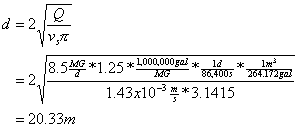
2. Settling Performance (25 points)
The town of Pleasant Valley has a secondary clarifier that is 30 m long and 15 m wide. If the typical wastewater flow is 3.5 MGD, what is the diameter of the largest bacterial particle (assume a density of 1.02 g/cm3) that will be not be retained and therefore discharged into the Pleasant River at 5°C?
First, calculate the overflow rate
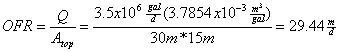
This will then be the critical settling velocity, So taking Stokes law and rearranging:
![]()
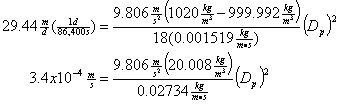
and then solving for Dp:
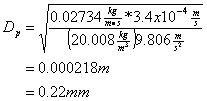
3. Short Answer (25 points)
a. Sketch a typical growth curve for a bacterial population in a batch culture, and describe the various phases (5 points)
See figure 5-10 in Mihelcic and related description
b. Sketch 3 different growth models, list their names and briefly describe how they differ. (5 points)
See book or class overheads. Models include: linear, exponential, logistic, monod
c. What are the factors that can limit bacterial growth? Explain. (5 points)
Carrying capacity, which might include such factors as population density, presence of toxins, availability of food (electron donor, and carbon source), temperature, pH, light energy (for phototrophs), disease.
d. List the 4 “Great Spheres” of living and non-living material, and briefly indicate what each encompasses (5 points)
Atmosphere, Hydrosphere, Lithosphere & Biosphere (see section 5.1 in Mihelcic for descriptions)
e. What are phytoplankton and zooplankton? (5 points)
These are microscopic plants and animals (respectively) that live in natural surface waters. They are free floating, and constitute an important food source for higher animals
4. Groundwater and contaminant flow (25
points)
Two groundwater wells are located 75 m apart within the same aquifer. Well #1 has a water level at 1273 ft above mean sea level. The second well’s water level is 1311 ft above mean sea level. The mean hydraulic conductivity in this aquifer is 0.18 m/day, and the porosity is 0.65. How long will it take the water to travel from Well #2 to Well #1?
First determine the Darcy’s velocity between the wells
![]()
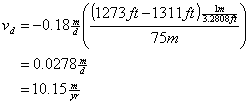
Then determine the true velocity between the wells
![]()
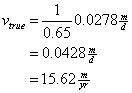
Now determine the time it takes for water to travel between the two wells
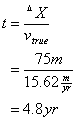
5. Microbial
Growth (25 points)
You’re operating a batch reactor. At the start (time =0) you have 0.85 mg/L of biomass. After two hours of operation you find that the biomass concentration is 1.40 mg/L. You know that this particular substrate you’re treating is characterized by a half-saturation coefficient of 12 mg/L. The starting substrate concentration was 9 mg/L and the final value at 2 hours was essentially unchanged.
a. What is the specific growth rate assuming simple exponential growth throughout?
From Equation 5-5:
![]()
![]()
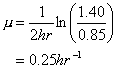
b. What is the maximum specific growth rate for this system?
![]()
so:
![]()
6. Activated Sludge Models (25 points)
You’re operating an activated sludge biological treatment system for a medium sized community. Your wastewater flow rate is 5.5 MGD. The influent BOD (S0) is 385 mg/L and your effluent value (S) is 7.5 mg/L. Your biological treatment system (e.g., aeration basin) has a total volume of 4,000 m3. Assume a yield coefficient of 0.55, and a decay coeffient of 0.06 d-1. Also assume that you will have a biomass concentration (X) in the aeration basin of 3,500 mg/L.
a. What is the solids retention time you will need to use to operate the system as described above?
From page 236:
![]()
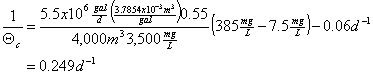
![]()
b. What will be the waste pumping rate (Qw) you will need to use? Assume that the waste (or recycle) solids concentration is 15,000 mg/L.
Recall:
![]()
so:
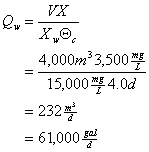
7. Reactor Kinetics #1 (25 points)
You’re using a CMFR to treat an industrial waste containing phenol. Aqueous chlorine reacts with phenol according to second order kinetics (first order in phenol and first order in chlorine). The observed second order rate constant for this reaction is 2.23x103 M-1min-1 at the temperature and pH of the wastewater (25 C, and pH 7.0). To accomplish this removal you’ve designed a CMFR reactor with a volume is 50 m3 and you’ve decided to add aqueous chlorine so that the concentration in the reactor is constant at 0.001 moles/L. If the volumetric flow rate at the inlet and outlet is 5000 m3/day and then inlet phenol concentration is 10-4 moles/L, what will the phenol concentration at the outlet be?
This may be viewed as a pseudo-first order reaction:
![]()
where: k=k2[Chlorine]=2.23x103M-1min-1(0.001M)=2.23min-1=3,210d-1
and now:
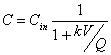
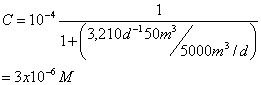
8. True/False. Indicate whether the following statements are true (T) or false (F). (25 points total; 2.5 points each)
|
1. |
T |
PFRs are
always more efficient that CMFRs for 1st order processes |
|
2. |
T |
The latent
heat of condensation is the heat released when a gas condenses to form a
liquid |
|
3. |
F |
Greenhouse
gases reflect light energy |
|
4. |
F |
Fick’s
first law describes advective flow |
|
5. |
F |
Stoke’s law
describes molecular diffusion |
|
6. |
T |
Darcy’s law
describes flow in porous media |
|
7. |
F |
The
lithosphere is a part of the biotic environment |
|
8. |
F |
Rotifers
are a form of algae |
|
9. |
T |
Protozoa
can exist in a cyst form |
|
10. |
F |
Most
primary producers are bacteria |
9. Reactor Kinetics #2 (25 points)
Northampton uses the Mountain Street Reservoir in Williamsburg for a portion of its water supply. The water is travels to Northampton in a 20 inch diameter main that stretches 5,310 ft. The City typically adds 3x10-5 moles/L (2.13 mg/L) of chlorine to the water as it enters the main. Assume that this level is not substantially diminished in concentration during the time it travels to Northampton, and assume that the flow approximates a plug flow reactor. Chlorine reacts with manganese by second-order kinetics (first order in chlorine and first order in manganese). The rate constant under these conditions (pH 7.0, 20 C) is 9 M-1s-1. If the flow from Mountain Street Reservoir is 1.5 MGD, and the raw water reduced manganese concentration is 2x10-6 moles/L (0.11 mg/L), what will the reduced manganese concentration be once the water reaches the end of the main?
For a PFR:
![]()
First let’s calculate the volume of the pipe:
![]()
Next let’s determine the pseudo-first order rate constant, k:
![]()
where:
![]()
now combining:
![]()
This is also equal to 29 ppb.
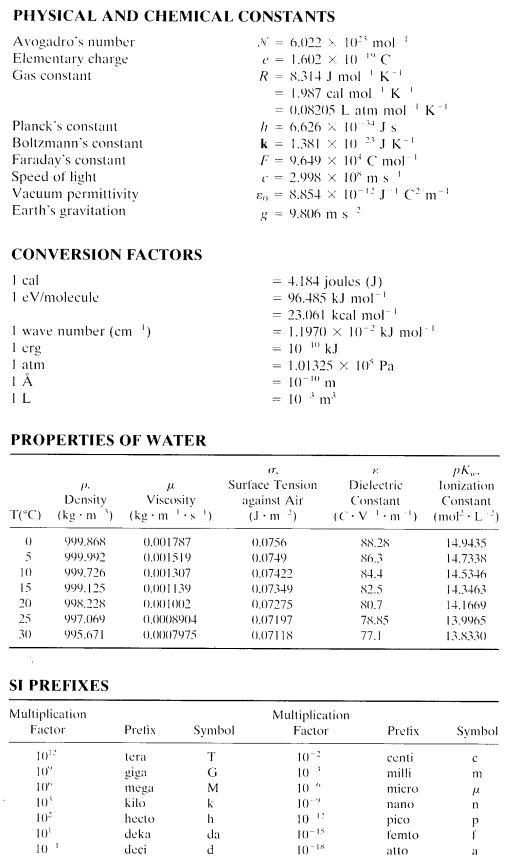
Appendix
Selected Chemical Constants
|
Element |
Symbol |
Atomic # |
Atomic Wt. |
Valence |
Electronegativity |
|
|
Aluminum |
Al |
13 |
26.98 |
3 |
1.47 |
|
|
Boron |
B |
5 |
10.81 |
3 |
2.01 |
|
|
Calcium |
Ca |
20 |
40.08 |
2 |
1.04 |
|
|
Carbon |
C |
6 |
12.01 |
2,4 |
2.50 |
|
|
Cerium |
Ce |
58 |
140.12 |
3,4 |
1.06 |
|
|
Helium |
He |
2 |
4.00 |
0 |
|
|
|
Holmiuum |
Ho |
67 |
164.93 |
3 |
1.10 |
|
|
Hydrogen |
H |
1 |
1.01 |
1 |
2.20 |
|
|
Magnesium |
Mg |
12 |
24.31 |
2 |
1.23 |
|
|
Manganese |
Mn |
25 |
54.94 |
2,3,4,6,7 |
1.60 |
|
|
Osmium |
Os |
76 |
190.2 |
2,3,4,8 |
1.52 |
|
|
Oxygen |
O |
8 |
16.00 |
2 |
3.50 |
|
|
Potassium |
K |
19 |
39.10 |
1 |
0.91 |
|
|
Sodium |
Na |
11 |
22.99 |
1 |
1.01 |
|
|
Sulfur |
S |
16 |
32.06 |
2,4,6 |
2.44 |
|
Selected Acidity Constants (Aqueous Solution, 25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
|
Sulfuric acid |
H2SO4=
H+ + HSO4- |
-3 |
|
|
Nitric acid |
HNO3 = H+ +
NO3- |
-0 |
|
|
Bisulfate ion |
HSO4-
= H+ + SO4-2 |
2 |
|
|
Phosphoric acid |
H3PO4 =
H+ + H2PO4- |
2.15 |
|
|
Hydrofluoric acid |
HF = H+ + F- |
3.2 |
|
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
|
Propionic acid |
C2H5COOH
= H+ + C2H5COO- |
4.87 |
|
|
Carbonic acid |
H2CO3 =
H+ + HCO3- |
6.35 |
|
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02 |
|
|
Dihydrogen phosphate |
H2PO4-
= H+ + HPO4-2 |
7.2 |
|
|
Hypochlorous acid |
HOCl = H+ + OCl- |
7.5 |
|
|
Ammonium ion |
NH4+
= H+ + NH3 |
9.24 |
|
|
Hydrocyanic acid |
HCN = H+ + CN- |
9.3 |
|
|
Phenol |
C6H5OH
= H+ + C6H5O- |
9.9 |
|
|
Bicarbonate ion |
HCO3-
= H+ + CO3-2 |
10.33 |
|
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |
|
|
Bisulfide ion |
HS- = H+ + S-2 |
13.9 |
|
Conversion factors:
1 gal = 3.7854x10-3 m3
1 ft = 0.3048 m
Other Constants from Mihelcic: