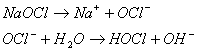|
CEE 370 |
|
Name _________________________________
Final Exam
December 21, 2006
Closed book, 3 sheets of notes allowed.
Show all work. Be neat, and box-in your answer.
If you cannot finish a problem (e.g., due to lack of time, or a missing formula), explain on your exam paper how you would have completed it.
 Answer:
Answer:
· Question #1 (20%)
·
 Question #2 (15%)
Question #2 (15%)
· one from the next three questions (#3 - # 5) (15%) ________________
· Question #6 (30%)
·
 Question
#7 (5%)
Question
#7 (5%)
· one from the last four questions #8 - #11 (15%) ________________
total is 100%
**********************************************************************
Answer both of the first two questions #1 & #2
1. Stoichiometry (20 points)
Aerobic heterotrophic bacteria can facilitate the following unbalanced biochemical transformation of glucose:
C6H12O6 + O2 ® CO2 + H2O
A. Which element (and in which chemical species) is being oxidized in this reaction and what is its initial and final oxidation state?
B. Which element (and in which chemical species) is being reduced in this reaction and what is its initial and final oxidation state?
C. Balance the glucose-oxygen reaction.
D. Determine the Theoretical Oxygen Demand (ThOD) of a 25 mg/L solution of glucose.
2. Reactor Kinetics (15 points)
A. A flow of 20 L/min of water is to be treated in a plug flow reactor (PFR). What reactor volume (L) is needed to achieve 95% removal of a contaminant with a first order decay rate of 0.20 min-1?
B. Calculate the volume needed if the water is to be treated for 95% removal in an ideal complete mix flow reactor (CMFR).
Answer one of the next three questions #3 - #5
3. Solid Waste (15 points)
A. How much sawdust (total weight) must be added to a waste sludge to lower the moisture content to 60% so that it can be composted? Assume you have 1000 kg of sludge (dry weight). The sludge (as discarded) is 12% dry solids, 88% moisture, and the sawdust is 75% dry solids, 25% moisture.
B. If the sludge were dewatered such that the total solids were 30% (rather than 12%) how much sawdust (total weight) would be needed for the same situation?
4. Hazardous Waste (15 points)
Chlorine may be used to destroy cyanide ion (CN-) in industrial wastes, producing nitrogen gas and bicarbonate. The chlorine is commonly added as sodium hypochlorite (NaOCl). The reactions that occur are as follows:
And:
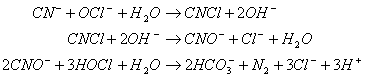
A. If you had 100 kg of cyanide, how many kilograms of sodium hypochlorite would be needed?
B. How many moles of sodium hydroxide (NaOH) or hydrochloric acid (HCl) would also be needed to neutralize the reaction mixture if you started with 1 mole of cyanide and the requisite amount of sodium hypochlorite?
5. Air Pollution & Hazardous Waste, short answer (15 points)
A. Describe at least two ways that particulate air contaminants can be removed from gaseous emissions. Be as specific as possible.
B. Describe how acidic gases can be removed from incinerator waste gases. Include a diagram with your description.
C. Describe two in-situ cleanup methods for site remediation of soil contaminated with hazardous wastes.
D. Describe the characteristics of a hazardous waste, and how a waste might be defined as "hazardous" under the law.
Answer questions #6 and #7
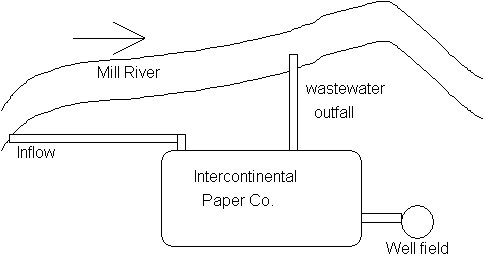
6. Water Quality Modeling (30 points)
The Intercontinental Paper Co. is discharging its wastewater directly into
the Mill River Mill River
Additional assumptions: BOD deoxygenation rate: k1(or kd) = 0.23 d-1
reaeration rate constant (k2 or kr) of 0.82 d-1
D.O saturation concentration = 9.5 mg/L
River flow velocity = 0.5 ft/s
7. BOD (5 points)
If the ultimate BOD of a wastewater is 350 mg/L and the kL is 0.25 d-1, what is the BOD5 ?
Answer one of the following four questions #8 - #11
8. Chemical Oxidation (15 points)
The oxidation of ferrous iron (Fe+2) by oxygen occurs in readily in water forming ferric hydroxide precipitate (Fe(OH)3(s)):
A. Write the two half cell reactions and balance each one.
B. Write the overall balanced reaction
C. How much oxygen (in mg/L) is required to oxidize 1.5 mg/L of ferrous iron and how much ferric hydroxide precipitate is formed?
9. Groundwater Flow (15 points)
The flow velocity (Darcy velocity) of water between two wells spaced 100 m apart has been documented at 0.003 m/day during a long dry period. During this time, the water surface elevation in the two wells was 335.93 m and 335.49 m. During periods of heavy recharge, the water levels rise to 342.85 m and 340.79 m respectively. What is the Darcy velocity between these wells under the heavy recharge period?
10. Biological Growth Kinetics (15 points)
You have been studying the performance of a CMFR biological reactor. Your wastewater supports a specific bacterial growth rate of 0.25 d-1. Under these conditions, the effluent substrate (waste) concentration is 0.4 mg/L. When you increase your wastewater strength such that the final substrate concentration leaving the CMFR is doubled to 0.8 mg/L, you observe a specific growth rate of 0.35 d-1. From these data estimate the two Monod coefficients, Ks and mmax.
11. Reactor Kinetics (15 points)
You have a wastewater flow rate of 1000 L/hr. To treat this wastewater you have constructed a concrete tank of 100,000 liters. The wastewater contains the deadly compound, 2-methyl-3-chloro-4-anisole sulfonate (MCAS). Kinetic studies have shown the MCAS to degrade by a 1st order reaction with a half-life of 40 hours.
a. What is the % removal of MCAS if the reactor is operated as a plug flow reaction (PRF)?
b. What is the % removal of MCAS if the reactor is operated as a completely mixed flow reactor (CMFR)?
Appendix
Some physical constants of Water:
|
Temp., oC |
Density, kg/m3 |
Viscosity, N-s/m2 |
Kinematic Viscosity, m2/s |
|
0 |
999.8 |
1.781x10-3 |
1.785x10-6 |
|
5 |
1000.0 |
1.518 x10-3 |
1.519x10-6 |
|
10 |
999.7 |
1.307 x10-3 |
1.306 x10-6 |
|
15 |
999.1 |
1.139 x10-3 |
1.139 x10-6 |
|
20 |
998.2 |
1.002 x10-3 |
1.003 x10-6 |
|
25 |
997.0 |
0.890 x10-3 |
0.893 x10-6 |
|
30 |
995.7 |
0.798 x10-3 |
0.800 x10-6 |
|
35 |
994.0 |
0.725 x10-3 |
0.729 x10-6 |
|
40 |
992.2 |
0.653 x10-3 |
0.658 x10-6 |
Selected Chemical Constants
|
Element |
Symbol |
Atomic # |
Atomic Wt. |
|
Electronegativity |
|
|
Aluminum |
Al |
13 |
26.98 |
3 |
1.47 |
|
|
Boron |
B |
5 |
10.81 |
3 |
2.01 |
|
|
Calcium |
Ca |
20 |
40.08 |
2 |
1.04 |
|
|
Carbon |
C |
6 |
12.01 |
2,4 |
2.50 |
|
|
Cerium |
Ce |
58 |
140.12 |
3,4 |
1.06 |
|
|
Chlorine |
Cl |
17 |
35.453 |
1 |
|
|
|
Holmiuum |
Ho |
67 |
164.93 |
3 |
1.10 |
|
|
Hydrogen |
H |
1 |
1.01 |
1 |
2.20 |
|
|
Iron |
Fe |
26 |
55.85 |
2,3 |
|
|
|
Magnesium |
Mg |
12 |
24.31 |
2 |
1.23 |
|
|
Manganese |
Mn |
25 |
54.94 |
2,3,4,6,7 |
1.60 |
|
|
Nitrogen |
N |
7 |
14.0067 |
3 |
|
|
|
Osmium |
Os |
76 |
190.2 |
2,3,4,8 |
1.52 |
|
|
Oxygen |
O |
8 |
16.00 |
2 |
3.50 |
|
|
Potassium |
K |
19 |
39.10 |
1 |
0.91 |
|
|
Sodium |
Na |
11 |
22.99 |
1 |
1.01 |
|
|
Sulfur |
S |
16 |
32.06 |
2,4,6 |
2.44 |
|
Useful conversion factors
![]()
![]()
![]()
1 ft = 0.305 m