|
CEE 370 |
Fall 2006 |
Exam #1
October 16, 2006
Closed Book, one sheet of notes allowed
Please answer any 4 of the following 9 questions. Each is worth 25 points. Show all work. Be neat, and box-in your answer.
1. Calculate the ThOD (in mg/L) of the following solutions: (25 points)
a. 10-3 moles/L of sodium nitrite, NaNO2
b. 3x10-3 moles/L of formic acid, HCOOH
c. 10-2 moles/L of ammonium carbonate, (NH4)2CO3
2. Perform a charge balance on the following water. As part of this balance determine the amount of unbalanced charge and its percentage of the total. What does this number tell you about the accuracy of the chemical analysis? (25 points)
|
Chemical Substance |
Conc. (mg/L) |
|
Na+ |
12.5 |
|
K+ |
1.1 |
|
Ca+2 |
39.3 |
|
Mg+2 |
3.5 |
|
NO3- |
8.8 |
|
SO4-2 |
12.4 |
|
Cl- |
33.9 |
|
HCO3- |
112.3 |
3. What is the TDS (in mg/L), and the ionic strength for the water in question #2? (25 points)
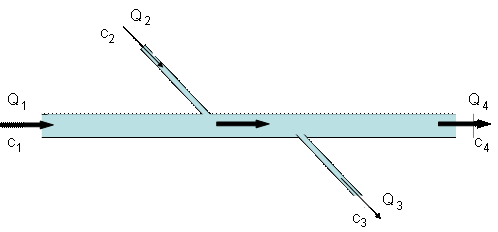
4. The diagram below shows a long deep-rock tunnel carrying water. There is one major inflow (Q1), one minor inflow (Q2), one minor outflow (Q3) and the major outflow (Q4), all but one of which are known. In addition, the concentration of chloride (a conservative substance) is known at 2 of the 4 inflow/outflow locations. Calculate the missing flow (Q3) and the missing chloride concentrations (c3 and c4) for this system (25 points)
|
Location |
Q (cfs) |
Chloride (mg/L) |
|
1 (main inflow) |
15 |
2 |
|
2 (side inflow) |
3 |
6 |
|
3 (side outflow) |
??? |
??? |
|
4 (main outflow) |
17 |
??? |
5. Use the following data to estimate the rate constant for the loss of chlorine dioxide in water. Assume the reaction is first order in chlorine dioxide (25 points)
|
Time (min) |
Concentration (µM) |
|
0 |
100 |
|
50 |
22 |
|
100 |
5 |
6. True/False. Indicate whether the following statements are true (T) or false (F). (25 points total; 2 points each; 1 free point)
|
1. |
|
The Arrhenius equation allows one to adjust rate constants from one temperature to another |
|
2. |
|
A triprotic acid has three times the strength as a monoprotic acid |
|
3. |
|
A conjugate base is what forms when an acid losses a proton
|
|
4. |
|
When an organic compound name ends in “ol”, it usually means the compound is an alcohol |
|
5. |
|
Catalysts accelerate chemical reactions by lowering the activation energy
|
|
6. |
|
When Gibbs Free Energy increases, the reaction will tend to go forward
|
|
7. |
|
Alkynes all have triple bonds
|
|
8. |
|
Henry’s law describes the relationship between partial pressure and dissolved concentration |
|
9. |
|
BTEX is a new type of dog food
|
|
10. |
|
Changes in ionic strength can cause shifts in chemical equilibria
|
|
11 |
|
An element of high electronegativity will share its bonding electrons equally with an element of low electronegativity |
|
12 |
|
Biochemical oxygen demand (BOD) differs from theoretical oxygen demand (ThOD) in that BOD only refers to oxygen demand from pharmaceuticals |
7. The half life for nonylphenol in groundwater is estimated to be 35 days. If the concentration of nonylphenol in a groundwater plume is currently 12 ppb, how long will it take for the concentration to drop below 1 ppb? (25 points)
8. The equilibrium constant for the following reaction is 10-10.3. What percent of the total carbonate is in the form of HCO3- at pH 11.5? (25 points)
HCO3- = CO3-2 + H+
9. You need to oxidize butyric acid (CH3CH2CH2COOH) completely to carbon dioxide (CO2) by addition of potassium dichromate (K2Cr2O7). After the reaction is complete, the chromium is in the trivalent form (Cr+3). How many mg/L of potassium dichromate are needed to completely oxidize 4.6 mg/L of butyric acid? (25 points).
Appendix
Selected Chemical Constants
|
Element |
Symbol |
Atomic # |
Atomic Wt. |
Valence |
Electronegativity |
|
|
Aluminum |
Al |
13 |
26.98 |
3 |
1.47 |
|
|
Boron |
B |
5 |
10.81 |
3 |
2.01 |
|
|
Calcium |
Ca |
20 |
40.08 |
2 |
1.04 |
|
|
Carbon |
C |
6 |
12.01 |
2,4 |
2.50 |
|
|
Chlorine |
Cl |
17 |
35.453 |
1,3,5,7 |
2.83 |
|
|
Chromium |
Cr |
24 |
52.00 |
many |
1.56 |
|
|
Helium |
He |
2 |
4.00 |
0 |
|
|
|
Holmiuum |
Ho |
67 |
164.93 |
3 |
1.10 |
|
|
Hydrogen |
H |
1 |
1.01 |
1 |
2.20 |
|
|
Magnesium |
Mg |
12 |
24.31 |
2 |
1.23 |
|
|
Manganese |
Mn |
25 |
54.94 |
2,3,4,6,7 |
1.60 |
|
|
Nitrogen |
N |
7 |
14.01 |
many |
3.07 |
|
|
Oxygen |
O |
8 |
16.00 |
2 |
3.50 |
|
|
Potassium |
K |
19 |
39.10 |
1 |
0.91 |
|
|
Sodium |
Na |
11 |
22.99 |
1 |
1.01 |
|
|
Sulfur |
S |
16 |
32.06 |
2,4,6 |
2.44 |
|
Selected Acidity Constants (Aqueous Solution, 25°C, I = 0)
|
NAME |
FORMULA |
pKa |
|
|
Hydrochloric acid |
HCl = H+ + Cl- |
-3 |
|
|
Sulfuric acid |
H2SO4= H+ + HSO4- |
-3 |
|
|
Nitric acid |
HNO3 = H+ + NO3- |
-0 |
|
|
Bisulfate ion |
HSO4- = H+ + SO4-2 |
2 |
|
|
Phosphoric acid |
H3PO4 = H+ + H2PO4- |
2.15 |
|
|
Hydrofluoric acid |
HF = H+ + F- |
3.2 |
|
|
Nitrous acid |
HNO2 = H+ + NO2- |
4.5 |
|
|
Acetic acid |
CH3COOH = H+ + CH3COO- |
4.75 |
|
|
Propionic acid |
C2H5COOH = H+ + C2H5COO- |
4.87 |
|
|
Carbonic acid |
H2CO3 = H+ + HCO3- |
6.35 |
|
|
Hydrogen sulfide |
H2S = H+ + HS- |
7.02 |
|
|
Dihydrogen phosphate |
H2PO4- = H+ + HPO4-2 |
7.2 |
|
|
Hypochlorous acid |
HOCl = H+ + OCl- |
7.5 |
|
|
Ammonium ion |
NH4+ = H+ + NH3 |
9.24 |
|
|
Hydrocyanic acid |
HCN = H+ + CN- |
9.3 |
|
|
Phenol |
C6H5OH = H+ + C6H5O- |
9.9 |
|
|
Bicarbonate ion |
HCO3- = H+ + CO3-2 |
10.33 |
|
|
Monohydrogen phosphate |
HPO4-2 = H+ + PO4-3 |
12.3 |
|
|
Bisulfide ion |
HS- = H+ + S-2 |
13.9 |
|